Slow axonal transport is the movement of cytoskeletal polymers and cytosolic protein complexes along axons at average rates on the order of millimeters per day, which corresponds to nanometers per second.
From: Encyclopedia of Neuroscience, 2009
Slow Axonal Transport
A. Brown, in Encyclopedia of Neuroscience, 2009
The Mechanism of Slow Axonal Transport
Current research on slow axonal transport is focused on addressing four central questions: (1) What are the cargo structures of slow components a and b, (2) what are the tracks along which they move, (3) what are the motors that move them, and (4) how is their movement regulated? The observation of the rapid intermittent movement of neurofilament and microtubule polymers in axons indicates that these cytoskeletal polymers are among the cargo structures of slow axonal transport, although this does not preclude the possibility that cytoskeletal subunit proteins may also move in an unassembled form. The form in which the many other proteins in slow axonal transport move remains unclear. One hypothesis is that they are transported by binding to (and effectively riding piggyback on) moving cytoskeletal polymers. The relatively simple protein composition of slow component a suggests that neurofilaments and microtubules could be the sole carrier structures for this rate component; all the proteins that move in slow component a are either integral parts of these cytoskeletal polymers or are known to associate with these polymers in vivo. The presence of actin in slow component b suggests that microfilaments could function as a carrier structure for this rate component, although it seems unlikely that the several hundred different proteins that move in slow component b all bind to microfilaments. More probably, many of these proteins form supramolecular complexes that, in turn, associate with the moving filaments or that associate with motor proteins independently of the moving filaments. However, this hypothesis is speculative because the movement of microfilaments and most other cytosolic proteins in axons has not yet been demonstrated.
The rapid rate of movement of neurofilaments and microtubules in axons indicates that they are transported by fast motors, perhaps similar to or even the same as the motors that move membranous organelles, but the identity of these motors and the tracks along which they move are not known. By analogy with the movement of membranous organelles, it seems likely that neurofilaments and microtubules and other cargoes of slow axonal transport move along either microtubule or microfilament tracks. For example, there is evidence that dynein may transport axonal microtubules anterogradely along microfilaments and that dynein and kinesin may transport axonal neurofilaments bidirectionally along microtubules. The frequency of neurofilament movement may be regulated by phosphorylation of the neurofilament proteins. Axonal neurofilaments can also interact with myosin Va, but the role of this interaction in neurofilament movement is presently unclear. Although much remains to be learned about the mechanism of movement, it seems likely that the cargo structures of slow axonal transport each interacts with a number of different motor proteins and that these motors act cooperatively to translocate and organize these polymers in both the longitudinal and radial dimensions of the axon.
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780080450469020003
Subcellular Organization of the Nervous System
Scott Brady, … Peter Brophy, in From Molecules to Networks (Third Edition), 2014
Slow Axonal Transport Moves Soluble Proteins and Cytoskeletal Structures
Slow axonal transport has two major components, both representing movement of cytoplasmic constituents (Fig. 2.8). Cytoplasmic elements in axonal transport move at rates comparable to the rate of neurite elongation. Slow Component a (SCa) is movement of cytoskeletal elements, primarily neurofilaments and microtubules; SCa rates typically range from 0.1–1 mm/day and newly synthesized cytoskeletal proteins may take >1000 days to reach the end of a meter-long axon. Slow Component b (SCb) is a complex and heterogeneous rate component, including hundreds of distinct polypeptides from cytoskeletal proteins such as actin (and sometimes tubulin) to soluble enzymes of intermediary metabolism (i.e., glycolytic enzymes). SCb moves at 2–4 mm/day and is the rate-limiting component for nerve growth or regeneration (Roy et al., 2007).
Figure 2.8. Slow axonal transport represents the delivery of cytoskeletal and cytoplasmic constituents to the periphery.
Cytoplasmic proteins are synthesized on free polysomes and organized for transport as cytoskeletal elements or macromolecular complexes (1). Microtubules are formed by nucleation at the microtubule-organizing center near the centriolar complex (2) and then released for migration into axons or dendrites. Slow transport appears to be unidirectional with no net retrograde component. Studies suggest that cytoplasmic dynein may move microtubules with their plus ends leading (3). Neurofilaments may move on their own or may hitchhike on microtubules (4). Once cytoplasmic structures reach their destinations, they are degraded by local proteases (5) at a rate that allows either growth (in the case of growth cones) or maintenance of steady-state levels. The different composition and organization of cytoplasmic elements in dendrites suggest that different pathways may be involved in delivery of cytoskeletal and cytoplasmic materials to dendrites (6). In addition, some mRNAs are transported into dendrites, but not into axons.
The coordinated movement of neurofilament and microtubule proteins provided strong evidence for the “structural hypothesis.” For example, in pulse-labeling experiments labeled neurofilament proteins move as a bell-shaped wave with little or no trailing of neurofilament protein (Baas and Buster, 2004). Neurofilament stability under physiological conditions indicates that soluble neurofilament subunit pools are negligible, so coherent transport of neurofilament triplet proteins implied a transport complex, i.e., neurofilaments. Similarly, coordinate transport of tubulin and MAPs made sense only if microtubules move, because MAPs do not interact with unpolymerized tubulin. The simplest explanation is that neurofilaments and microtubules move as discrete cytological structures, but this idea was controversial for many years.
Development of fluorescently tagged neurofilament or microtubule subunits and methods for visualizing these structures in living cells resolved this issue by documenting movements of individual microtubules and neurofilaments in neurites of cultured neurons (Brown, 2003). Direct observations of individual microtubule or neurofilament segments indicated that they move down axons as assembled polymers. Video images of fluorescently tagged microtubules or neurofilaments reveal discontinuous movements, with long pauses punctuated by brief, rapid translocations at 1–2 μm/sec. Due to long pauses, average rates are 2–3 orders of magnitude slower than instantaneous velocities (Baas and Buster, 2004). Remarkably, dynein plays a major role in slow axonal transport of microtubules and neurofilaments (He et al., 2005).
Studies on transport of neurofilament proteins indicated that little or no degradation occurs until neurofilaments reach nerve terminals, where they are degraded rapidly. Comparable results were obtained with microtubule proteins. Differential metabolism appears to be a key to targeting of cytoplasmic and cytoskeletal proteins. Proteins with slow degradative rates accumulate, reaching higher steady-state levels. Altering degradation rates changes that steady-state concentration, so enrichment of actin in presynaptic terminals is due to slower turnover of actin than neurofilaments and tubulin. As a result, inhibiting calpain causes neurofilament accumulation in terminals. Differential turnover may involve specific proteases or posttranslational modifications that affect susceptibility to degradation. Regardless, cytoplasmic proteins are degraded in the distal axon and do not return in retrograde axonal transport.
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780123971791000026
Subcellular Organization of the Nervous System
Scott T. Brady, … Peter J. Brophy, in Fundamental Neuroscience (Fourth Edition), 2013
Slow Axonal Transport Moves Soluble Components and Cytoskeletal Structures
Slow axonal transport has two major components, both representing the movement of cytoplasmic constituents (Fig. 4.7). The cytoplasmic and cytoskeletal elements of the axon in axonal transport move at rates at least two orders of magnitude more slowly than fast transport. Slow component a is composed largely of cytoskeletal proteins, neurofilaments, and microtubule protein. Slow component b is a complex and heterogeneous rate component, including hundreds of distinct polypeptides ranging from cytoskeletal proteins such as actin (and tubulin in some nerves) to soluble enzymes of intermediary metabolism (such as glycolytic enzymes). Many characteristics of axonal transport have been described, and these characteristics provide the foundation for our understanding of mechanisms.
Neurofilaments and microtubules move as discrete cytological structures (Baas & Buster, 2004). Studies on the transport of neurofilament protein indicate that little degradation or metabolism occurs until neurofilaments reach nerve terminals, where they are degraded rapidly. Comparable results have been obtained in studies labeling microtubule protein by radioactivity or fluorescence. Under favorable conditions, movement of individual microtubules can be detected in neurites or growth cones. Both radiolabeling studies and direct observations of individual microtubules indicate that all microtubules and neurofilaments move down the axon, but the motor protein involved is uncertain. Differential metabolism appears to be a key to the targeting of cytoplasmic and cytoskeletal proteins. Proteins with slow degradative rates accumulate and reach higher steady-state concentrations. Alteration of degradation rates changes the steady-state concentration of a protein. The concentration of actin in presynaptic terminals is explained by a slower turnover in terminals relative to neurofilament proteins and tubulin, and inhibition of calpain causes neurofilament rings to appear in presynaptic terminals. Differential turnover may be accomplished by specific proteases or posttranslational modifications that affect the susceptibility to degradation.
The coherent movement of neurofilaments and microtubule proteins provides strong evidence for the “structural hypothesis.” For example, pulse-labeling experiments show that radiolabeled neurofilament proteins move as a bell-shaped wave with little or no trailing of neurofilament protein. This fits with the observed stability of neurofilaments under physiological conditions. Thus, any soluble pool of neurofilament subunits is negligible. Similarly, the coherent transport of tubulin and MAPs makes sense only if microtubules are moved because MAPs do not interact with unpolymerized tubulin.
A striking demonstration of microtubule movement can be seen with fluorescent analogs of tubulin (Brown, 2003). When local segments of an axon are photoactivated, short fluorescent microtubule segments can be seen to move down the growing neurite. These microtubules move rapidly (1–2 µm/sec) with frequent pauses that produce a net anterograde transport of microtubules at 1–2 mm/day and similar rates are observed for neurofilaments. When studies of axonal transport using radiolabeled amino acids are combined with direct observations of individual microtubules or neurofilaments by video microscopy, there is little doubt that microtubules and neurofilaments can and do move in the axon as intact, individual cytoskeletal elements.
More recently, similar methods have been used to document the transport of classically soluble, cytoplasmic proteins like glycolytic enzymes and synuclein (Roy, Winton, Black, Trojanowski, & Lee, 2007). As with microtubules and neurofilaments, these movements are intermittent and rapid. The extended pauses lead to a net rate of anterograde transport of approximately 2–4 mm/day, consistent with rates obtained in pulse-chase radiolabel studies.
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780123858702000044
Subcellular Organization of the Nervous System: Organelles and Their Functions
Scott Brady, … Peter Brophy, in From Molecules to Networks, 2004
Slow Axonal Transport Moves Soluble Components and Cytoskeletal Structure
Slow axonal transport has two major components, both representing movement of cytoplasmic constituents (Fig. 2.11). The cytoplasmic and cytoskeletal elements of the axon in axonal transport move at rates at least two orders of magnitude more slowly than fast transport. Slow component a is composed largely of cytoskeletal proteins, neurofilaments, and microtubule protein. Slow component b is a complex and heterogeneous rate component, including hundreds of distinct polypeptides ranging from cytoskeletal proteins such as actin (and tubulin in some nerves) to soluble enzymes of intermediary metabolism (such as the glycolytic enzymes). Many characteristics of axonal transport have been described, and these characteristics provide the foundation for our understanding of mechanisms.
FIGURE 2.11. Slow axonal transport represents the delivery of cytoskeletal and cytoplasmic constituents to the periphery. Cytoplasmic proteins are synthesized on free polysomes and organized for transport as cytoskeletal elements or macromolecular complexes (1). The microtubules are formed by nucleation at the microtubule-organizing center near the centriolar complex (2) and then released for migration into the axon or dendrites. The molecular mechanisms are not as well understood as those for fast axonal transport, but slow transport appears to be unidirectional with no retrograde component. Recent studies suggest that motors like cytoplasmic dynein may interact with the axonal membrane cytoskeleton to move the microtubules with their plus ends leading (3). Neurofilaments do not appear able to move on their own, but may hitchhike on the microtubules (4). Other cytoplasmic proteins may do the same or they may be moved by other motors. Once cytoplasmic structures reach their destinations, they are degraded by local proteases (5) at a rate that allows either growth (in the case of growth cones) or maintenance of steady-state levels. The different composition and organization of the cytoplasmic elements in dendrites suggest that different pathways may be involved in delivery of cytoskeletal and cytoplasmic materials to the dendrite (6). In addition, some mRNAs are transported into the dendrites, but not into axons.
Neurofilaments and microtubules move as discrete cytological structures (Brady, 1992). Recent studies on transport of neurofilament protein indicate that little degradation or metabolism occurs until neurofilaments reach nerve terminals, where they are rapidly degraded. Comparable results have been obtained in studies labeling microtubule protein by radioactivity or fluorescence. Under favorable conditions, movement of individual microtubules can be detected in neurites or growth cones. Both radiolabeling studies and direct observations of individual microtubules indicate that all microtubules and neurofilaments move down the axon, but the motor protein involved is uncertain. Differential metabolism appears to be a key to the targeting of cytoplasmic and cytoskeletal proteins. Proteins with slow degradative rates accumulate and reach higher steady-state concentrations. Alteration of degradation rates changes the steady-state concentration of a protein. Concentration of actin in presynaptic terminals is explained by slower turnover in terminals relative to neurofilament proteins and tubulin, and inhibition of calpain causes neurofilament rings to appear in presynaptic terminals. Differential turnover may be accomplished by specific proteases or posttranslational modifications that affect susceptibility to degradation.
The coherent movement of neurofilaments and microtubule proteins provides strong evidence for the “structural hypothesis.” For example, pulse-labeling experiments show that radiolabeled neurofilament proteins move as a bell-shaped wave with little or no trailing of neurofilament protein. This fits with the observed stability of neurofilaments under physiological conditions, which suggests that any soluble pool of neurofilament subunits is negligible. Similarly, the coherent transport of tubulin and MAPs makes sense only if microtubules are moved, because MAPs do not interact with unpolymerized tubulin.
A striking demonstration of microtubule movement can be seen with fluorescent analogs of tubulin (Tanaka and Kirschner, 1991; Reinsch et al, 1991). Tubulin labeled with caged fluorescein can be injected into one cell of a fertilized Xenopus oocyte at the two-cell stage. Such tubulins are fluorescent only after photoactivation. The injected oocyte is then allowed to develop into an embryo. The injected tubulin equilibrates with endogenous tubulin and is incorporated into the microtubules of all cells derived from the original injected cell. Because protein synthesis is minimal in early cell divisions of embryonic development, the labeled tubulin is reused by daughter cells until diluted out by newly synthesized tubulin. When early embryonic neurons are cultured, the caged fluorescent tubulin may be photoactivated and visualized.
When local segments of an axon are photoactivated, patches of fluorescent tubulin can be seen to move down the growing neurite. The fluorescent patches remain discrete during movements in the anterograde direction at slow transport rates. Observations of full microtubules can also be made in embryonic Xenopus neurons by using rhodaminelabeled tubulin. In favorable areas of axons and growth cones, individual fluorescent microtubules can be visualized. Such microtubules can be seen to move down axons and into growth cones. The forces are sufficient to bend these microtubules in conjunction with growth cone movements. When studies of axonal transport using radiolabels are combined with direct observations of individual microtubules by video microscopy, there is little doubt that microtubules and neurofilaments can and do move in the axon as intact, individual cytoskeletal elements.
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780121486600500032
Slow Axonal Transport☆
A. Brown, in Reference Module in Biomedical Sciences, 2014
The Kinetics of Slow Axonal Transport
When considering the rate of slow axonal transport, it is important to make a distinction between measurements made on fast and slow timescales. In radioisotopic pulse-labeling experiments, which are performed on a timescale of days or weeks, each protein conveyed by slow axonal transport moves out along the axon in the form of a unimodal bell-shaped wave that spreads as it moves distally. The rate of slow axonal transport is generally quoted as the rate of movement of the peak of the wave, which represents the modal transport rate, although the width and spreading of the waves clearly indicate that the proteins actually move at a range of rates. The modal rate differs in different neuronal cell types and may also vary spatially (along axons) as well as temporally (during development, maturation, and aging), but it is generally around 0.2–1 mm day− 1 (0.002–0.02 μm s− 1) for slow component a and 2–8 mm day− 1 (0.02–0.09 μm s− 1) for slow component b (Table 2).
In contrast to the radioisotopic pulse-labeling experiments, direct observations on cultured neurons using fluorescence microscopy on a timescale of seconds or minutes indicate that cytoskeletal and cytosolic proteins actually move very rapidly, at rates of up to several micrometers per second, and that these movements are also transient or infrequent (Figure 5). Thus, the bell-shaped waves that characterize slow axonal transport in radioisotopic pulse-labeling experiments can be considered to represent the distribution of hundreds or thousands of pulse-labeled cargoes whose individual movements and pauses are summed over the days, weeks, or months that they spend traveling down the axon. Whether the movement is considered to be fast or slow depends on the timescale of the observations.
Figure 5. Kinetics of neurofilament transport on fast and slow timescales: (a) timescale of weeks to months as revealed by radioisotopic pulse-labeling in rat spinal motor neurons in vivo; (b) timescale of seconds to minutes, as revealed by live cell fluorescence imaging in cultured nerve cells. In (a), the pulse of radiolabeled neurofilament proteins moves out along the sciatic nerve in the form of a bell-shaped wave that spreads as it moves distally. Note that this wave represents the movement of a large ensemble of neurofilaments in a population of axons. The graph in (b) represents a single neurofilament moving in an anterograde direction along a single axon. The filament was tracked by time-lapse imaging (as described in Figure 3). Each point represents the position of the leading end of the neurofilament along the axon in one 5 s interval. Both anterogradely and retrogradely moving neurofilaments exhibit a similar behavior, characterized by bouts of rapid movement interrupted by pauses of varying durations.
(a) Data from Hoffman PN, Griffin JW, Gold BG, and Price DL (1985) Slowing of neurofilament transport and the radial growth of developing nerve fibers. Journal of Neuroscience 5: 2920–2929, courtesy of Paul Hoffman. (b) Data from Wang L and Brown A (2001) Rapid intermittent of axonal neurofilaments observed by fluorescence photobleaching. Molecular Biology of the Cell 12: 3257–3267.
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780128012383047656
Axonal Transport
Gerardo A. Morfini, … Gerardo Morfini, in Basic Neurochemistry (Eighth Edition), 2012
Properties of slow axonal transport suggest molecular mechanisms
Information about molecular mechanisms underlying slow axonal transport is relatively limited. They are energy dependent and require intact MTs. Accordingly, transport of NFs can be pharmacologically uncoupled from MT transport without eliminating slow transport (Griffin et al., 1995). Recent imaging studies in cultured neurons confirmed these findings, further showing that transport of NFs (Francis et al., 2005) and various proteins in SCb does not depend on actin (Roy et al., 2008). In contrast, pharmacological agents that disrupt MTs appear to block slow transport of all components. While this does not rule out a role for the MF cytoskeleton in slow transport movements, MTs appear to be required for the transport of other elements of the cytoskeleton.
The macroscopic transport velocity rates measured by radiolabeling experiments should not be taken to reflect maximum rates of the motors involved. As with mitochondrial transport, the net rate velocity of slow component proteins reflects both the rate of actual movement and the fraction of a time interval that a structure is moving (Brady, 2000). The large size and elongated shape of cytoskeletal structures and their potential for many interactions means that net displacements are discontinuous. If a structure is moving at a speed of 2 µm/sec, but on average only moves at that rate for 1 out of every 100 seconds, then the average rate for the structure will translate to an net rate of only 0.02 µm/sec (Baas & Buster, 2004).
Recently, methods for direct visualization of fluorescently tagged MTs and NFs in cultured neurons have been developed (Brown, 2003). In such studies, relatively short MT or NF segments can be seen to move as rapidly as MBOs moving in fast axonal transport, although they move much less frequently. Integration of these infrequent rapid movements of MTs and NFs over time gives a net rate of approximately 1–2 mm/day instead of the 200–400 mm/day seen for MBOs in mammalian nerve. Other studies permitted visualization of microtubules nucleated at the microtubule organizing center and being translocated toward the cell periphery. The combination of studies of axonal transport using radiolabels and direct observations of individual MTs or NFs with video microscopy provides strong experimental evidence that MTs and NFs can and do move in the axon as intact cytoskeletal structures. As discussed below, there are still questions about the specific motors and mechanisms underlying these movements.
Like membrane proteins, cytoplasmic and cytoskeletal proteins are differentially distributed in neurons and glia. Progress has been made toward identification of targeting mechanisms and some general principles have begun to emerge. Since cytoplasmic constituents move only in the anterograde direction, a key mechanism for targeting of cytoplasmic and cytoskeletal proteins appears to be differential metabolism (Brady, 1993). Concentration of actin and other proteins in presynaptic terminals can be explained by slower turnover in the presynaptic terminal relative to NF proteins and tubulin. Proteins with slow degradative rates in the terminal would accumulate and reach a higher steady-state concentration. Thus, alteration of degradation rates for a protein can change the rate of accumulation for that protein. For example, some protease inhibitors cause the appearance of neurofilament rings in affected presynaptic terminals (Roots, 1983).
Although slow axonal transport of cytoskeletal proteins has received the most attention, all other cytoplasmic proteins must be delivered to specific neuronal compartments of the neuron as well. Many of these have been defined as part of the “cytosol” or soluble fraction that results from biochemical fractionation. These include the enzymes of glycolysis and regulatory proteins like calmodulin and HSC70 (Brady, 1993). However, in pulse-chase radiolabel studies, soluble proteins move down the axon as regularly and systematically as cytoskeletal proteins. Again, this coherent transport of hundreds of different polypeptides appears consistent with the Structural Hypothesis and indicates a higher level of organization of cytoplasmic proteins than has been traditionally assumed (Lasek & Brady, 1982). Such organization is likely necessary to facilitate interactions with motor proteins and targeting mechanisms and to assure a reliable delivery of all required proteins to the axon at appropriate stoichometries.
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780123749475000080
Presynaptic Regulation by Liprins
K. Miller, D. Van Vactor, in Encyclopedia of Neuroscience, 2009
Kinesins, Liprin, and Presynaptic Vesicle Precursor Transport
There are two major subdivisions of axonal transport: fast and slow. Soluble cytoskeletal proteins such as tau, kinesin, dynein, myosin, and tubulin are transported at a rate of approximately 1 mm day–1 by slow axonal transport. Membrane-bound proteins, associated with organelles such as presynaptic precursor vesicles, are transported at a rate of approximately 1 μm s–1 by fast axonal transport. The kinesin family of molecular motors moves soluble proteins and organelles away from the cell body, and dynein moves these materials toward the cell body. When kinesin-1 is disrupted, axonal clogs that contain synaptic components occur along axons and synaptic transmission is disrupted.
While substantial evidence suggests that liprin-α plays a direct role in determining the form and function of synapses, recent data also reveal that liprin-α is involved in the fast axonal transport of presynaptic vesicle precursors (PVPs). PVPs contain the raw materials for the assembly of synapses, and while they are thought to be transported primarily by kinesin-3 (Unc-104, Kif1a), kinesin-1 (conventional kinesin) family members may also play a role in their transport. Since axonal accumulations of endogenous synaptotagmin similar to those found in kinesin heavy chain (Khc) alleles are also observed in mutants lacking Drosophila liprin-α, we speculate that liprin-α is involved in the transport of a subset of synaptic components and that this subset may be transported by more than one family of kinesin.
At one time is was thought that there were molecules whose sole purpose was to link kinesins to cargos, but it now appears that most adaptors are scaffolding proteins that interact with multiple proteins and do not serve as simple linkers. Liprin-α binds to kinesin-1 and kinesin-3. Yet, the balance of the evidence is against liprin-α acting as a simple linker between PVPs and kinesins. When it is disrupted, the transport of PVPs is not halted. Instead, it shifts the probability of transport from anterograde (kinesin-mediated) to retrograde (dynein-mediated) transport. Thus, liprin-α is not required for the movement of PVPs per se. Instead of a simple linker, liprin-α appears as a modulator of axonal transport.
One of the most basic questions in neurobiology is how some types of neuronal components are trafficked to dendrites, and others are selectively transported into axons. Liprin-α is part of a complex that targets postsynaptic components to dendrites and involves GRIP, AMPARs, and kinesin-1. Of note, there are splice variants of liprin-α that lack the GRIP-binding domain. It is tempting to speculate that this differential targeting between axons and dendrites may involve variations in splicing. Yet further work will be required to determine if and how liprin-α could help to selectively guide components into either axonal or dendritic compartments. While several lines of evidence suggest that liprin-α regulates transport, a great deal of work remains to provide a satisfying molecular answer as to how this occurs.
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780080450469017745
Axonal Transport: Properties, Mechanisms, and Role in Nerve Disease
STEPHEN BRIMIJOIN, in Peripheral Neuropathy (Fourth Edition), 2005
Overview of Toxicant-Induced Neuropathies
In 1969, Pleasure and associates reported marked reduction of slow axonal transport of radiolabeled protein in the central processes of sensory neurons of cats with acrylamide neuropathy.180 Since that original study, both fast and slow anterograde transport have been measured by various methods in many experimental neuropathies (Table 18-1). Minor reductions in fast-transport velocity have been encountered in the neuropathies induced by zinc pyridinethione (ZPT),147 acrylamide,26 and vincristine,26 although some studies have reported normal velocities in the same conditions.217 More substantial slowing of fast axonal transport, up to 36%, has been seen in hexacarbon neuropathy.152 Overall, however, one is struck by the extent to which fast anterograde transport seems to persist at full speed despite substantial axonal pathology. Few if any of the toxicant-induced neuropathies involve gross defects of fast anterograde transport. Some more pronounced effects on slow anterograde transport in several different toxic neuropathies are detailed subsequently.
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780721694917500211
Organizational Cell Biology
Y. Tanaka, N. Hirokawa, in Encyclopedia of Cell Biology, 2016
Discovery of Kinesin and Kinesin Superfamily Proteins
In a historic metabolic labeling experiment of eyeballs, protein transport toward optic nerve axons was classified into several categories, including fast and slow axonal transport (Grafstein and Forman, 1980). These transport mechanisms function at maximum speeds of 240 mm per day (group I), 34–68 mm per day (group II), 4–8 mm per day (group III), 2–4 mm per day (group IV, slow component b), and 0.7–1.1 mm per day (group V, slow component a). Structural proteins and metabolic enzymes are primarily transported by the slow mechanisms, while membrane organelles are primarily transported by the fast mechanism. High resolution electron microscopy described short filamentous structures, which are probable candidates for motor proteins that link microtubules to organelles (Hirokawa, 1982). In fact, conventional kinesin, now defined as kinesin-1 holoenzyme, was identified in 1985 via biochemical purification (Brady, 1985; Vale et al., 1985) as one of the enzymes that is responsible for anterograde fast axonal transport along the microtubule cytoskeleton. One kinesin-1 holoenzyme consists of two kinesin heavy chains (KHCs; KIF5A, 5B, or 5C) that display microtubule-activated motor ATPase activity and two kinesin light chains (KLCs) that serve as cargo connectors. Later, MAP1C, also referred to as cytoplasmic dynein, was identified as a major retrograde motor (Paschal et al., 1987). We first visualized purified kinesin-1 molecules via electron microscopy, resulting in an appearance that was identical to the organelle-microtubule cross-linking structure in axons that were specifically decorated by anti-kinesin-1 antibodies (Hirokawa et al., 1989; Figure 1).
Figure 1. Structures of kinesin in vitro and in vivo. (a) High magnification image of rotary-shadowed kinesin. One or two globular domains are visible at the right end of each molecule, whereas less well-defined, but generally fan-shaped, domains can be detected at the opposite ends. (b) Quick-freeze, deep-etch electron micrograph of rat brain neurites. Many of the cross-bridges contain globular bulges where they appear to contact microtubules (see arrows). Bars, 100 nm.
Reproduced with permission from Hirokawa, N., Pfister, K.K., Yorifuji, H., et al., 1989. Submolecular domains of bovine brain kinesin identified by electron microscopy and monoclonal antibody decoration. Cell 56, 867–878.
The physiological relevance of kinesin-1 in intracellular transport was revealed by genetics studies of fungi, Drosophila (Hurd et al., 1996), and mice (Tanaka et al., 1998). For example, kinesin-1 depletion via gene targeting in mice revealed striking perinuclear clustering of its mitochondrial cargo in extra-embryonic yolk sac cells (Tanaka et al., 1998). This perinuclear clustering was proposed to be due to an imbalance of bidirectional transport in these cells, providing genetic evidence that kinesin can significantly alter the intracellular localization of organelles. Concurrently, positional cloning of yeast and fungal cell division mutants revealed the existence of kinesin-like proteins that shared several amino acid sequences with kinesin-1, such as KAR3 in yeast (Meluh and Rose, 1990). Based on RT-PCR cloning using degenerate primers corresponding to consensus sequences from a mouse brain cDNA library, our group first identified all 45 genes for the mammalian kinesin superfamily proteins (KIFs) (Miki et al., 2001; Nakagawa et al., 1997; Aizawa et al., 1992).
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780123944474200606
Axonal Transport and ALS
E.L.F. Holzbaur, in Encyclopedia of Neuroscience, 2009
Slow axonal transport
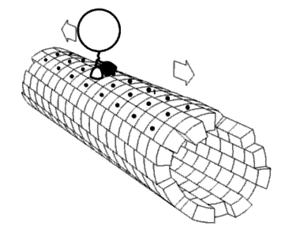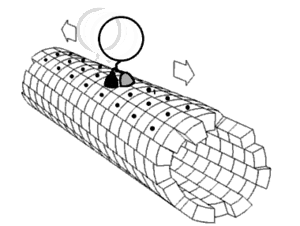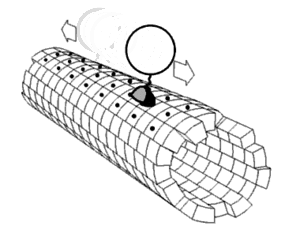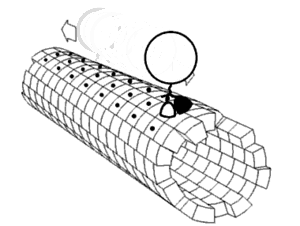
Neurofilaments and other cytoskeletal polymers are transported down the axon at a rate of 0.2–8 mm day−1, in a process known as ‘slow’ axonal transport. This transport is orders of magnitude slower than the transport of vesicular cargos in ‘fast’ axonal transport, at rates of ∼200–400 mm day−1. For a long time, the mechanisms driving the slow anterograde transport of neurofilaments were unclear. However, recent progress has shown that slow transport along the axon involves the same microtubule motor proteins that drive fast transport. The key difference is that for cargo such as neurofilaments that undergo slow transport, rapid excursions in either the anterograde or retrograde direction are punctuated by prolonged pauses. Both the frequent changes in direction and the extended pauses contribute to an overall net slow outward movement.
The reasons why cytoskeletal polymers are transported in such an apparently inefficient mechanism remain to be determined, but one possibility is that the oppositely oriented forces, applied simultaneously by plus and minus end-directed motors, result in a continuous tension on the cargo. In the case of neurofilaments, this tension may keep the long, flexible filaments stretched out along microtubules, and therefore may counteract aggregation. Support for this hypothesis comes from the observation that knockdown of dynein expression or inhibition of dynein/dynactin function leads to the distal accumulation of neurofilaments along neuronal processes.
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780080450469007117
Аксональный транспорт
Аксональный
транспорт (аксоток) – это перемещение
веществ от тела нейрона в отростки
(антероградный
аксоток) и в обратном направлении
(ретроградный
аксоток).
Различают медленный
аксональный ток веществ (1-5 мм в сутки)
и быстрый
(до 1-5 м в сутки). Обе транспортные системы
присутствуют как в аксонах, так и в
дендритах.
Аксональный транспорт обеспечивает
единство нейрона. Он создаёт постоянную
связь между телом нейрона (трофическим
центром) и отростками. Основные
синтетические процессы идут в
перикарионе. Здесь сосредоточены
необходимые для этого органеллы. В
отростках синтетические процессы
протекают слабо.
Антероградная
быстрая система
транспортирует к нервным окончаниям
белки и органеллы, необходимые для
синаптических функций (митохондрии,
фрагменты мембран, пузырьки, белки-ферменты,
участвующие в обмене нейромедиаторов,
а также предшественники нейромедиаторов).
Ретроградная
система
возвращает в перикарион использованные
и поврежденные мембраны и белки для
деградации в лизосомах и обновления,
приносит информацию о состоянии
периферии, факторы роста нервов.
Медленный транспорт
– это антероградная система, проводящая
белки и другие вещества для обновления
аксоплазмы зрелых нейронов и
обеспечения роста отростков при их
развитии и регенерации.
Ретроградный транспорт может иметь
значение в патологии. За счёт него
нейротропные вирусы (герпеса, бешенства,
полиомиелита) могут перемещаться с
периферии в центральную нервную систему.
Нейроглия (глиоциты)
Глиоиты выполняют
в нервной ткани вспомогательные функции:
опорную, разграничительную, трофическую,
секреторную и защитную. Они поддерживают
постоянство среды вокруг нейронов.
Клетки нейроглии делятся на две группы:
макроглию и
микроглию.
Клетки макроглии бывают трёх типов:
эпендимоциты, астроциты и олигодендроциты
(рис. 8-4).
Эпендимоциты.
Выстилают
каналы и желудочки спинного и головного
мозга, по которым циркулирует
спинномозговая жидкость (ликвор).
Эти клетки напоминают однослойный
призматический эпителий. На апикальных
концах эпендимоцитов расположены
реснички, помогающие движению
спинномозговой жидкости. Через
апикальные концы эпендимоциты могут
выделять биологически активные вещества,
которые с ликвором разносятся по
всему мозгу. От базальных концов
эпиндимоцитов отходят отростки, которые
могут идти через весь мозг и образуют
на его поверхности глиальную мембрану.
В желудочках мозга находятся сосудистые
сплетения.
Они покрыты специализированными
эпендимоцитами, участвующими в
образовании ликвора.
Астроциты.
Различают протоплазматические и
волокнистые астроциты (рис. 8-4).
Протоплазматические
астроциты
имеют короткие толстые отростки. Они
расположены в сером веществе мозга,
выполняют разграничительную и трофическую
функции. Волокнистые
астроциты находятся
в белом веществе, имеют многочисленные
тонкие длинные отростки, которые
оплетают кровеносные сосуды мозга,
образуя периваскулярные глиальные
пограничные мембраны. Их отростки также
изолируют синапсы. Таким образом, они
изолируют нейроны и кровеносные сосуды
и участвуют в образовании
гемато-энцефалического барьера,
обеспечивают обмен веществ между кровью
и нейронами. Они также участвуют в
образовании оболочек мозга и выполняют
опорную функцию (образуют каркас мозга).
Олигодендроциты
имеют мало
отростков (рис. 8-4), окружают нейроны,
выполняя трофическую (участие в питании
нейронов) и разграничительную функции.
Олигодендроциты расположенные вокруг
тел нейронов называются мантийными
глиоцитами.
Олигодендроциты, расположенные в
периферической нервной системе и
образующие оболочки вокруг отростков
нейронов, называют леммоцитами
(шванновскими
клетками).
Микроглия (глиальные
макрофаги; рис. 8-4). Образуются из
костномозговых предшественников
моноцитов. Покоящиеся микроглиоциты
имеют короткие ветвящиеся отростки.
Под действием микроорганизмов и продуктов
распада нервной ткани они активируются,
теряют отростки, округляются и
превращаются в «зернистые шары»
(реактивная микроглия). При этом они,
как макрофаги, уничтожают разрушенные
нервные и глиальные клетки.
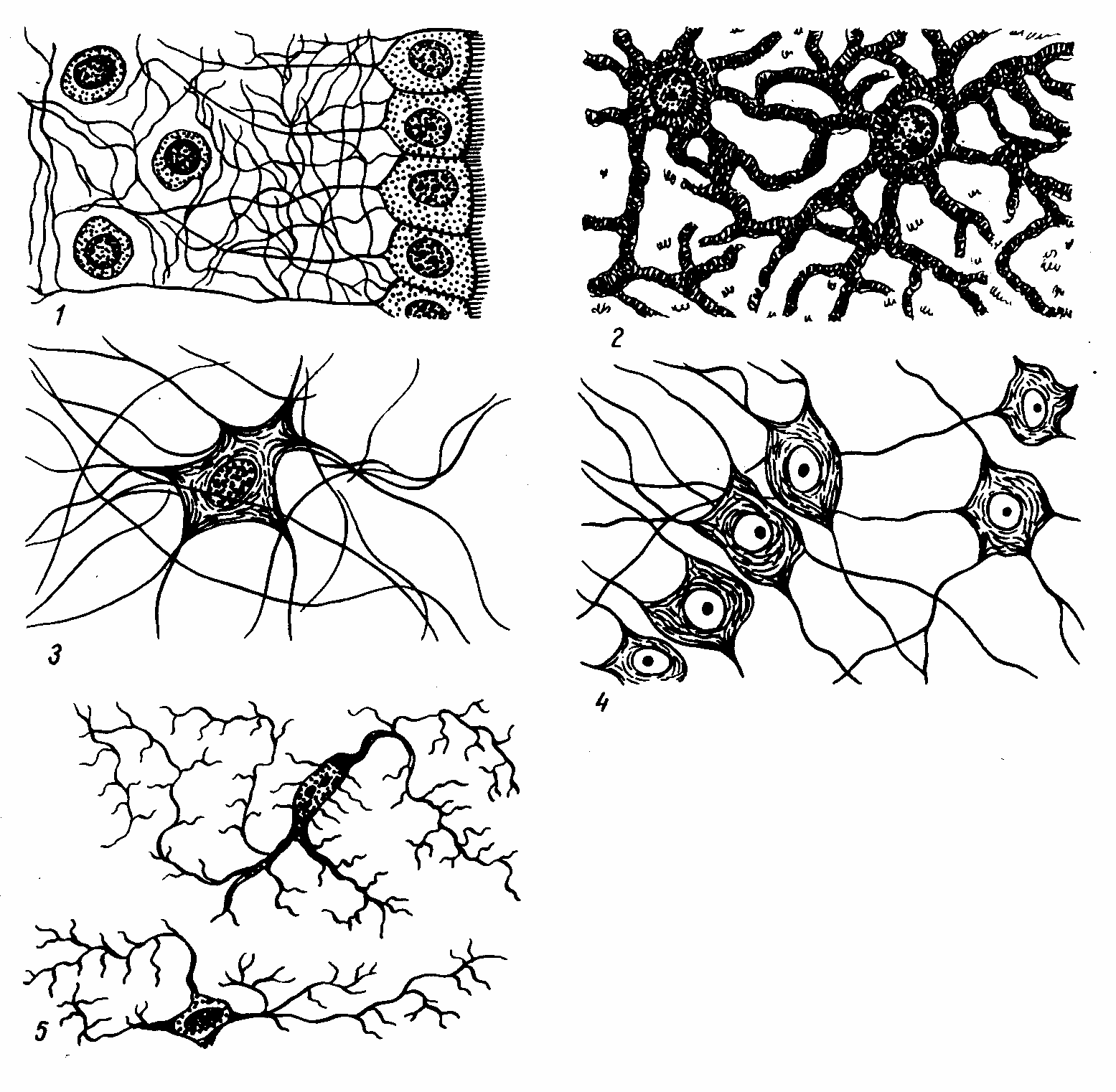
Рис. 8-4. Схема
глиоцитов различных видов. 1.
Эпендимоциты. 2. Протоплазматические
астроциты. 3. Волокнистые астроциты.
4. Олигодендроциты. 5. Микроглия. (По Т.
Н. Радостиной).
From Wikipedia, the free encyclopedia
Axonal transport, also called axoplasmic transport or axoplasmic flow, is a cellular process responsible for movement of mitochondria, lipids, synaptic vesicles, proteins, and other organelles to and from a neuron’s cell body, through the cytoplasm of its axon called the axoplasm.[1] Since some axons are on the order of meters long, neurons cannot rely on diffusion to carry products of the nucleus and organelles to the end of their axons. Axonal transport is also responsible for moving molecules destined for degradation from the axon back to the cell body, where they are broken down by lysosomes.[2]
Dynein, a motor protein responsible for retrograde axonal transport, carries vesicles and other cellular products toward the cell bodies of neurons. Its light chains bind the cargo, and its globular head regions bind the microtubule, «inching» along it.
Movement toward the cell body is called retrograde transport and movement toward the synapse is called anterograde transport.[3][4]
Mechanism[edit]
The vast majority of axonal proteins are synthesized in the neuronal cell body and transported along axons. Some mRNA translation has been demonstrated within axons.[5][6] Axonal transport occurs throughout the life of a neuron and is essential to its growth and survival. Microtubules (made of tubulin) run along the length of the axon and provide the main cytoskeletal «tracks» for transportation. Kinesin and dynein are motor proteins that move cargoes in the anterograde (forwards from the soma to the axon tip) and retrograde (backwards to the soma (cell body)) directions, respectively. Motor proteins bind and transport several different cargoes including mitochondria, cytoskeletal polymers, autophagosomes, and synaptic vesicles containing neurotransmitters.
Axonal transport can be fast or slow, and anterograde (away from the cell body) or retrograde (conveys materials from axon to cell body).
Fast and slow transport[edit]
Vesicular cargoes move relatively fast (50–400 mm/day) whereas transport of soluble (cytosolic) and cytoskeletal proteins takes much longer (moving at less than 8 mm/day).[7] The basic mechanism of fast axonal transport has been understood for decades but the mechanism of slow axonal transport is only recently becoming clear, as a result of advanced imaging techniques.[8] Fluorescent labeling techniques (e.g. fluorescence microscopy) have enabled direct visualization of transport in living neurons. (See also: Anterograde tracing.)
Recent studies have revealed that the movement of cytoskeletal «slow» cargoes is actually rapid but unlike fast cargoes, they pause frequently, making the overall transit rate much slower. The mechanism is known as the «Stop and Go» model of slow axonal transport, and has been extensively validated for the transport of the cytoskeletal protein neurofilament.[9] The movement of soluble (cytosolic) cargoes is more complex, but appears to have a similar basis where soluble proteins organize into multi-protein complexes that are then conveyed by transient interactions with more rapidly moving cargoes moving in fast axonal transport.[10][11][12] An analogy is the difference in transport rates between local and express subway trains. Though both types of train travel at similar velocities between stations, the local train takes much longer to reach the end of the line because it stops at every station whereas the express makes only a few stops on the way.
Anterograde transport[edit]
Anterograde (also called «orthograde») transport is movement of molecules/organelles outward, from the cell body (also called soma) to the synapse or cell membrane.
The anterograde movement of individual cargoes (in transport vesicles) of both fast and slow components along the microtubule[4] is mediated by kinesins.[2] Several kinesins have been implicated in slow transport,[8] though the mechanism for generating the «pauses» in the transit of slow component cargoes is still unknown.
There are two classes of slow anterograde transport: slow component a (SCa) that carries mainly microtubules and neurofilaments at 0.1-1 millimeters per day, and slow component b (SCb) that carries over 200 diverse proteins and actin at a rate of up to 6 millimeters per day.[8] The slow component b, which also carries actin, are transported at a rate of 2-3 millimeters per day in retinal cell axons.
During reactivation from latency, the herpes simplex virus (HSV) enters its lytic cycle, and uses anterograde transport mechanisms to migrate from dorsal root ganglia neurons to the skin or mucosa that it subsequently affects.[13]
A cargo-receptor for anterograde transport motors, the kinesins, has been identified as the amyloid precursor protein (APP), the parent protein that produces the senile plaques found in Alzheimer’s disease.[14] A 15-amino acid peptide in the cytoplasmic carboxyl terminus of APP binds with high affinity to conventional kinesin-1 and mediates transport of exogenous cargo in the giant axon of the squid.[15]
Manganese, a contrast agent for T1-weighted MRI, travels by anterograde transport after stereotaxic injection into the brain of experimental animals and thereby reveals circuitry by whole brain MR imaging in living animals, as pioneered by Robia Pautler, Elaine Bearer and Russ Jacobs. Studies in kinesin-light chain-1 knockout mice revealed that Mn2+ travels by kinesin-based transport in the optic nerve and in the brain. Transport in both hippocampal projections and in the optic nerve also depends on APP.[16] Transport from hippocampus to forebrain is decreased in aging and destination is altered by the presence of Alzheimer’s disease plaques.[17]
Retrograde transport[edit]
Retrograde transport shuttles molecules/organelles away from axon termini toward the cell body. Retrograde axonal transport is mediated by cytoplasmic dynein, and is used for example to send chemical messages and endocytosis products headed to endolysosomes from the axon back to the cell.[2] Operating at average in vivo speeds of approximately 2 μm/sec,[18][19] fast retrograde transport can cover 10-20 centimeters per day.[2]
Fast retrograde transport returns used synaptic vesicles and other materials to the soma and informs the soma of conditions at the axon terminals. Retrograde transport carries survival signals from the synapse back to the cell body, such as the TRK, the nerve growth factor receptor.[20] Some pathogens exploit this process to invade the nervous system. They enter the distal tips on an axon and travel to the soma by retrograde transport. Examples include tetanus toxin and the herpes simplex, rabies, and polio viruses. In such infections, the delay between infection and the onset of symptoms corresponds to the time needed for the pathogens to reach the somata.[21] Herpes simplex virus travels both ways in axons depending on its life cycle, with retrograde transport dominating polarity for incoming capsids.[22]
Consequences of interruption[edit]
Whenever axonal transport is inhibited or interrupted, normal physiology becomes pathophysiology, and an accumulation of axoplasm, called an axonal spheroid, may result. Because axonal transport can be disrupted in a multitude of ways, axonal spheroids can be seen in many different classes of diseases, including genetic, traumatic, ischemic, infectious, toxic, degenerative and specific white matter diseases called leukoencephalopathies. Several rare neurodegenerative diseases are linked to genetic mutations in the motor proteins, kinesin and dynein, and in those cases, it is likely that axonal transport is a key player in mediating pathology.[23][24] Dysfunctional axonal transport is also linked to sporadic (common) forms of neurodegenerative diseases such as Alzheimer’s and Parkinson’s.[8] This is mainly due to numerous observations that large axonal accumulations are invariably seen in affected neurons, and that genes known to play a role in the familial forms of these diseases also have purported roles in normal axonal transport. However, there is little direct evidence for involvement of axonal transport in the latter diseases, and other mechanisms (such as direct synaptotoxicity) may be more relevant.
Arrest of axoplasmic flow at the edge of ischemic areas in vascular retinopathies leads to swelling of nerve fibres, which give rise to soft exudates or cotton-wool patches.
Since the axon depends on axoplasmic transport for vital proteins and materials, injury, such as diffuse axonal injury, which interrupts the transport, will cause the distal axon to degenerate in a process called Wallerian degeneration. Cancer drugs that interfere with cancerous growth by altering microtubules (which are necessary for cell division) damage nerves because the microtubules are necessary for axonal transport.
Infection[edit]
The rabies virus reaches the central nervous system by retrograde axoplasmic flow.[25] The tetanus neurotoxin is internalised at the neuromuscular junction through binding the nidogen proteins and is retrogradely transported towards the soma in signaling endosomes.[26] Neurotropic viruses, such the herpesviruses, travel inside axons using cellular transport machinery, as has been shown in work by Elaine Bearer’s group.[27][28] Other infectious agents are also suspected of using axonal transport.[29] Such infections are now thought to contribute to Alzheimer’s disease and other neurodegenerative neurological disorders.[30][31]
See also[edit]
- Intraflagellar transport
References[edit]
- ^ Sabry J, O’Connor TP, Kirschner MW (June 1995). «Axonal transport of tubulin in Ti1 pioneer neurons in situ». Neuron. 14 (6): 1247–56. doi:10.1016/0896-6273(95)90271-6. PMID 7541635.
- ^ a b c d Oztas E (2003). «Neuronal Tracing» (PDF). Neuroanatomy. 2: 2–5. Archived (PDF) from the original on 2005-10-25.
- ^ Karp G, van der Geer P (2005). Cell and molecular biology: concepts and experiments (4th ed.). John Wiley. p. 344. ISBN 978-0-471-46580-5.
- ^ a b Bear MF, Connors BW, Paradso MA (2007). Neuroscience : exploring the brain (3rd ed.). Lippincott Williams & Wilkins. p. 41. ISBN 978-0-7817-6003-4.
- ^ Giustetto M, Hegde AN, Si K, Casadio A, Inokuchi K, Pei W, Kandel ER, Schwartz JH (November 2003). «Axonal transport of eukaryotic translation elongation factor 1alpha mRNA couples transcription in the nucleus to long-term facilitation at the synapse». Proceedings of the National Academy of Sciences of the United States of America. 100 (23): 13680–5. Bibcode:2003PNAS..10013680G. doi:10.1073/pnas.1835674100. PMC 263873. PMID 14578450.
- ^ Si K, Giustetto Si K, Giustetto M, Etkin A, Hsu R, Janisiewicz AM, Miniaci MC, Kim JH, Zhu H, Kandel ER (December 2003). «A neuronal isoform of CPEB regulates local protein synthesis and stabilizes synapse-specific long-term facilitation in aplysia». Cell. 115 (7): 893–904. doi:10.1016/s0092-8674(03)01021-3. PMID 14697206. S2CID 15552012.
- ^ Maday, Sandra; Twelvetrees, Alison E.; Moughamian, Armen J.; Holzbaur, Erika L.F. (October 2014). «Axonal Transport: Cargo-Specific Mechanisms of Motility and Regulation». Neuron. 84 (2): 292–309. doi:10.1016/j.neuron.2014.10.019. PMC 4269290. PMID 25374356.
- ^ a b c d Roy S, Zhang B, Lee VM, Trojanowski JQ (January 2005). «Axonal transport defects: a common theme in neurodegenerative diseases». Acta Neuropathologica. 109 (1): 5–13. doi:10.1007/s00401-004-0952-x. PMID 15645263. S2CID 11635065.
- ^ Brown A (March 2003). «Axonal transport of membranous and nonmembranous cargoes: a unified perspective». The Journal of Cell Biology. 160 (6): 817–21. doi:10.1083/jcb.200212017. PMC 2173776. PMID 12642609.
- ^ Scott DA, Das U, Tang Y, Roy S (May 2011). «Mechanistic logic underlying the axonal transport of cytosolic proteins». Neuron. 70 (3): 441–54. doi:10.1016/j.neuron.2011.03.022. PMC 3096075. PMID 21555071.
- ^ Roy S, Winton MJ, Black MM, Trojanowski JQ, Lee VM (March 2007). «Rapid and intermittent cotransport of slow component-b proteins». The Journal of Neuroscience. 27 (12): 3131–8. doi:10.1523/JNEUROSCI.4999-06.2007. PMC 6672457. PMID 17376974.
- ^ Kuznetsov AV (2011). «Analytical solution of equations describing slow axonal transport based on the stop-and-go hypothesis». Central European Journal of Physics. 9 (3): 662–673. Bibcode:2011CEJPh…9..662K. doi:10.2478/s11534-010-0066-0.
- ^ Holland DJ, Miranda-Saksena M, Boadle RA, Armati P, Cunningham AL (October 1999). «Anterograde transport of herpes simplex virus proteins in axons of peripheral human fetal neurons: an immunoelectron microscopy study». Journal of Virology. 73 (10): 8503–11. doi:10.1128/JVI.73.10.8503-8511.1999. PMC 112870. PMID 10482603.
- ^ Satpute-Krishnan P, DeGiorgis JA, Conley MP, Jang M, Bearer EL (October 2006). «A peptide zipcode sufficient for anterograde transport within amyloid precursor protein». Proceedings of the National Academy of Sciences of the United States of America. 103 (44): 16532–7. Bibcode:2006PNAS..10316532S. doi:10.1073/pnas.0607527103. PMC 1621108. PMID 17062754.
- ^ Seamster PE, Loewenberg M, Pascal J, Chauviere A, Gonzales A, Cristini V, Bearer EL (October 2012). «Quantitative measurements and modeling of cargo-motor interactions during fast transport in the living axon». Physical Biology. 9 (5): 055005. Bibcode:2012PhBio…9e5005S. doi:10.1088/1478-3975/9/5/055005. PMC 3625656. PMID 23011729.
- ^ Gallagher JJ, Zhang X, Ziomek GJ, Jacobs RE, Bearer EL (April 2012). «Deficits in axonal transport in hippocampal-based circuitry and the visual pathway in APP knock-out animals witnessed by manganese enhanced MRI». NeuroImage. 60 (3): 1856–66. doi:10.1016/j.neuroimage.2012.01.132. PMC 3328142. PMID 22500926.
- ^ Bearer EL, Manifold-Wheeler BC, Medina CS, Gonzales AG, Chaves FL, Jacobs RE (October 2018). «Alterations of functional circuitry in aging brain and the impact of mutated APP expression». Neurobiology of Aging. 70: 276–290. doi:10.1016/j.neurobiolaging.2018.06.018. PMC 6159914. PMID 30055413.
- ^ Gibbs KL, Kalmar B, Sleigh JN, Greensmith L, Schiavo G (January 2016). «In vivo imaging of axonal transport in murine motor and sensory neurons». Journal of Neuroscience Methods. 257: 26–33. doi:10.1016/j.jneumeth.2015.09.018. PMC 4666412. PMID 26424507.
- ^ Sleigh J, Schiavo G (2016). «Older but not slower: aging does not alter axonal transport dynamics of signalling endosomes in vivo». Matters. 2 (6). doi:10.19185/matters.201605000018.
- ^ Cui B, Wu C, Chen L, Ramirez A, Bearer EL, Li WP, Mobley WC, Chu S (August 2007). «One at a time, live tracking of NGF axonal transport using quantum dots». Proceedings of the National Academy of Sciences of the United States of America. 104 (34): 13666–71. Bibcode:2007PNAS..10413666C. doi:10.1073/pnas.0706192104. PMC 1959439. PMID 17698956.
- ^ Saladin, Kenneth. Anatomy and Physiology: The Unity of Form and Function. Sixth. New York : McGraw-Hill, 2010. 445. Print.
- ^ Bearer EL, Breakefield XO, Schuback D, Reese TS, LaVail JH (July 2000). «Retrograde axonal transport of herpes simplex virus: evidence for a single mechanism and a role for tegument». Proceedings of the National Academy of Sciences of the United States of America. 97 (14): 8146–50. Bibcode:2000PNAS…97.8146B. doi:10.1073/pnas.97.14.8146. PMC 16684. PMID 10884436.
- ^ Maday S, Twelvetrees AE, Moughamian AJ, Holzbaur EL (October 2014). «Axonal transport: cargo-specific mechanisms of motility and regulation». Neuron. 84 (2): 292–309. doi:10.1016/j.neuron.2014.10.019. PMC 4269290. PMID 25374356.
- ^ Sleigh JN, Rossor AM, Fellows AD, Tosolini AP, Schiavo G (December 2019). «Axonal transport and neurological disease». Nat Rev Neurol. 15 (12): 691–703. doi:10.1038/s41582-019-0257-2. PMID 31558780. S2CID 203437348.
- ^ Mitrabhakdi E, Shuangshoti S, Wannakrairot P, Lewis RA, Susuki K, Laothamatas J, Hemachudha T (November 2005). «Difference in neuropathogenetic mechanisms in human furious and paralytic rabies». Journal of the Neurological Sciences. 238 (1–2): 3–10. doi:10.1016/j.jns.2005.05.004. PMID 16226769. S2CID 25509462.
- ^ Bercsenyi K, Schmieg N, Bryson JB, Wallace M, Caccin P, Golding M, Zanotti G, Greensmith L, Nischt R, Schiavo G (November 2014). «Tetanus toxin entry. Nidogens are therapeutic targets for the prevention of tetanus» (PDF). Science. 346 (6213): 1118–23. doi:10.1126/science.1258138. PMID 25430769. S2CID 206560426.
{{cite journal}}: CS1 maint: url-status (link) - ^ Satpute-Krishnan P, DeGiorgis JA, Bearer EL (December 2003). «Fast anterograde transport of herpes simplex virus: role for the amyloid precursor protein of alzheimer’s disease». Aging Cell. 2 (6): 305–18. doi:10.1046/j.1474-9728.2003.00069.x. PMC 3622731. PMID 14677633.
- ^ Cheng SB, Ferland P, Webster P, Bearer EL (March 2011). «Herpes simplex virus dances with amyloid precursor protein while exiting the cell». PLOS ONE. 6 (3): e17966. Bibcode:2011PLoSO…617966C. doi:10.1371/journal.pone.0017966. PMC 3069030. PMID 21483850.
- ^ Bearer EL, Satpute-Krishnan P (September 2002). «The role of the cytoskeleton in the life cycle of viruses and intracellular bacteria: tracks, motors, and polymerization machines». Current Drug Targets. Infectious Disorders. 2 (3): 247–64. doi:10.2174/1568005023342407. PMC 3616324. PMID 12462128.
- ^ Itzhaki RF, Lathe R, Balin BJ, Ball MJ, Bearer EL, Braak H, et al. (2016). «Microbes and Alzheimer’s Disease». Journal of Alzheimer’s Disease. 51 (4): 979–84. doi:10.3233/JAD-160152. PMC 5457904. PMID 26967229.
- ^ «No place like asphalt for these hardy microbes». New Scientist. 206 (2757): 15. 2010. doi:10.1016/s0262-4079(10)60991-8.
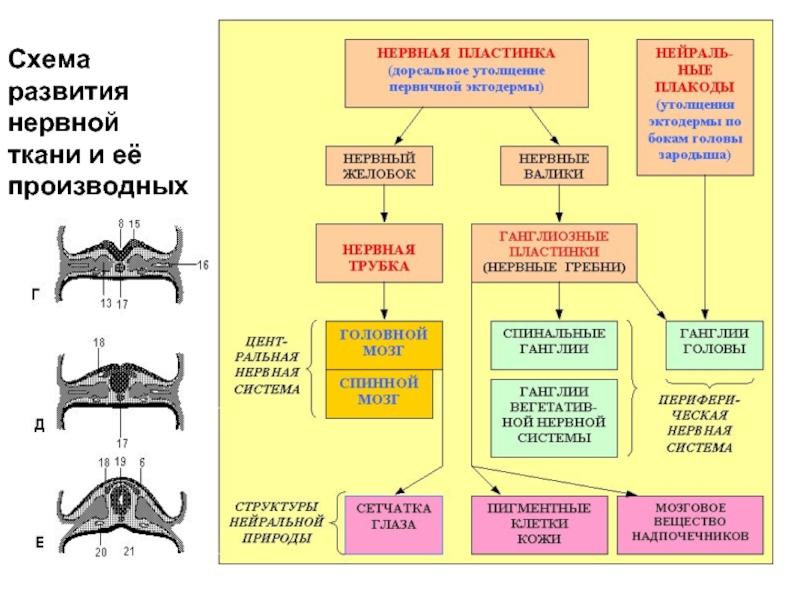
Слайд 2Схема развития нервной ткани и её производных
Слайд 3Сантья́го Рамо́н-и-Каха́ль (Santiago Ramón y Cajal)
(1852 — 1934)
Испанский врач
и гистолог, лауреат Нобелевской премии по физиологии медицине в 1906 году «В знак признания трудов о структуре нервной системы»
Ками́лло Го́льджи (Camillo Golgi) (1843 —1926)
Итальянский врач и учёный, лауреат Нобелевской премии по физиологии и медицине в 1906 году «В знак признания трудов о структуре нервной системы»

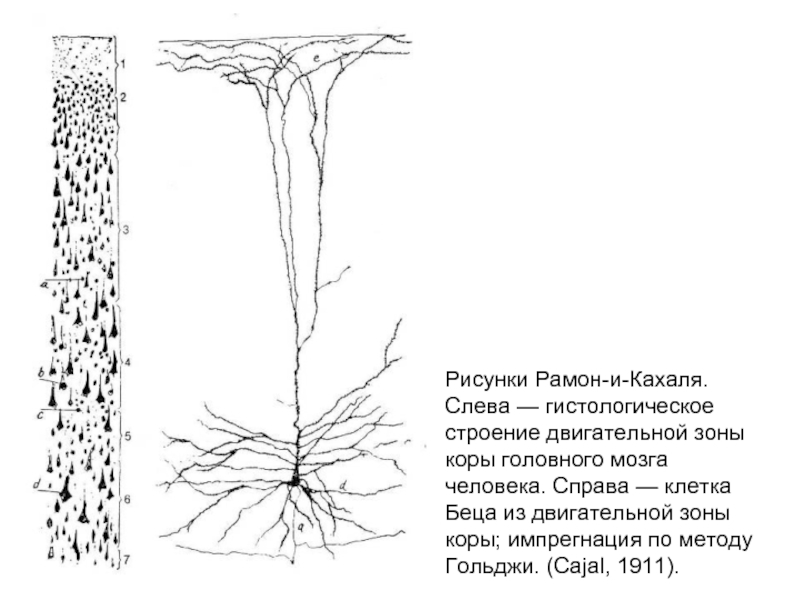
Слайд 4Рисунки Рамон-и-Кахаля.
Слева — гистологическое строение двигательной зоны коры головного мозга человека.
Справа — клетка Беца из двигательной зоны коры; импрегнация по методу Гольджи. (Cajal, 1911).
Слайд 9Основные типы нейронов :
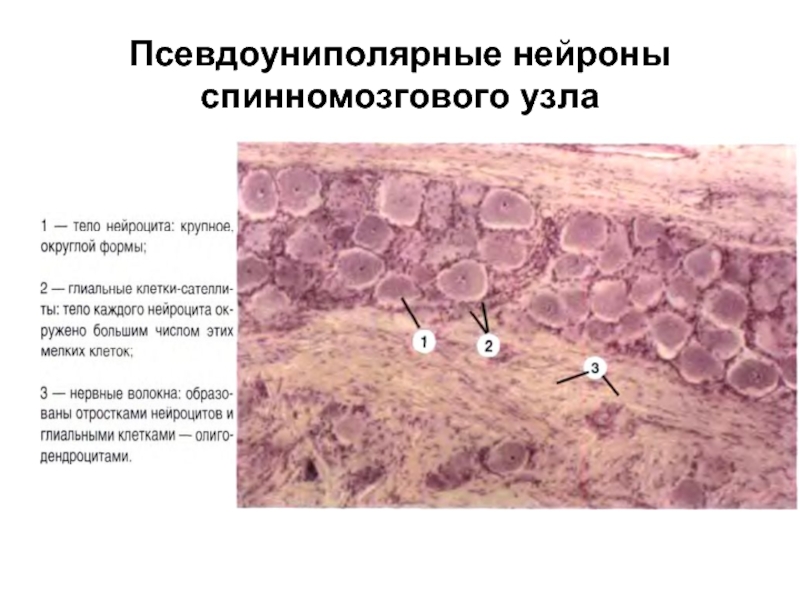
Униполярные. Встречаются только в эмбриональном периоде.
Псевдоуниполярные. Клетки, от
тела которых отходит только один отросток. На самом деле при выходе из сомы этот отросток разделяется на два: аксон и дендрит. Расположены в сенсорных ганглиях: спинномозговых и ганглиях головы.
Биполярные нейроны — это клетки, которые имеют один аксон и один дендрит. Присутствуют в сетчатке глаза и спиральном ганглии внутреннего уха
Мультиполярные нейроны имеют один аксон и множество дендритов. К такому типу нейронов принадлежит большинство нейронов ЦНС. Исходя из особенностей формы этих клеток их делят на веретенообразные, корзинчатые, звездчатые, пирамидные и др. Только в коре головного мозга насчитывается до 60 вариантов форм тел нейронов.

Слайд 10Псевдоуниполярные нейроны спинномозгового узла
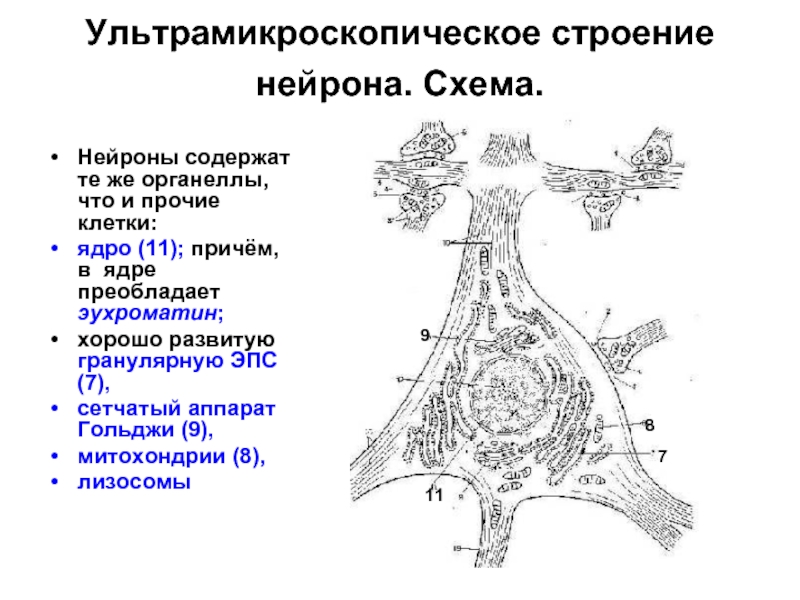
Слайд 11Ультрамикроскопическое строение нейрона. Схема.
Нейроны содержат те же органеллы, что и
прочие клетки:
ядро (11); причём, в ядре преобладает эухроматин;
хорошо развитую гранулярную ЭПС (7),
сетчатый аппарат Гольджи (9),
митохондрии (8),
лизосомы
11
7
9
8
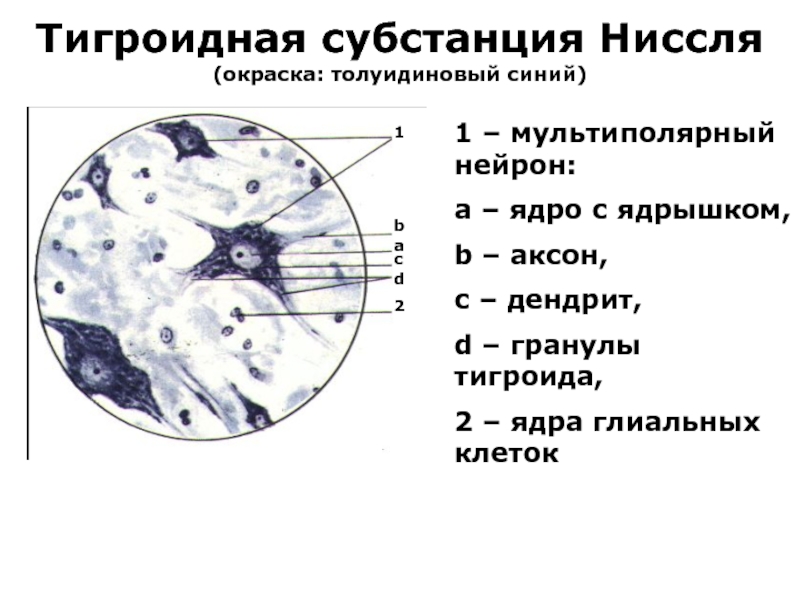
Слайд 12Тигроидная субстанция Нисcля
(окраска: толуидиновый синий)
1 – мультиполярный нейрон:
a – ядро
с ядрышком,
b – аксон,
c – дендрит,
d – гранулы тигроида,
2 – ядра глиальных клеток
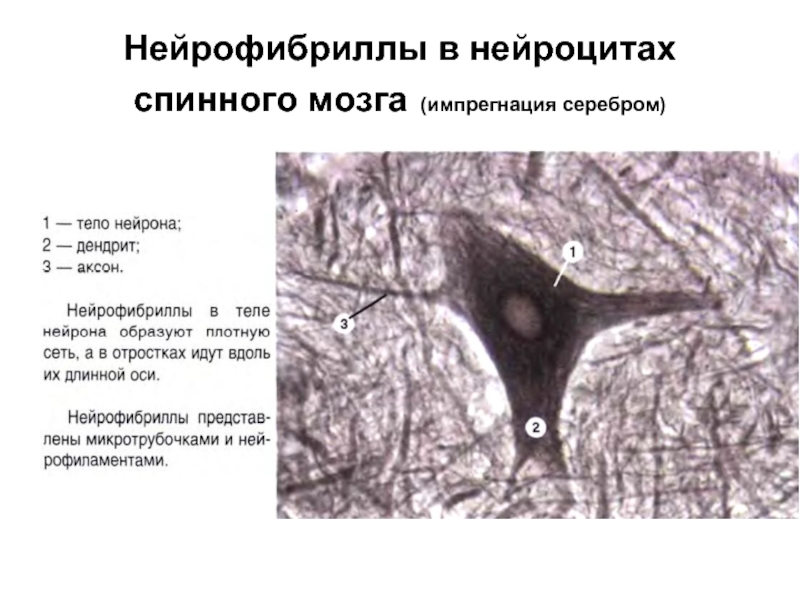
Слайд 13Нейрофибриллы в нейроцитах спинного мозга (импрегнация серебром)
Слайд 14Токи нейроплазмы
1. медленный ток (транспорт) по аксонам в прямом направлении —
со скоростью 1-3 мм/сутки (метаболиты, кислород, белки, нейрогормоны);
2. быстрый ток по аксонам в прямом направлении — 100-1000 мм/сутки (компоненты медиаторов);
3. ток по дендритам в прямом направлении — 75 мм/сутки (ацетилхолинэстераза к постсинаптической мембране);
4. ретроградный ток по аксонам и дендритам (конечные продукты обмена)(вирусы герпеса, бешенства и др.).

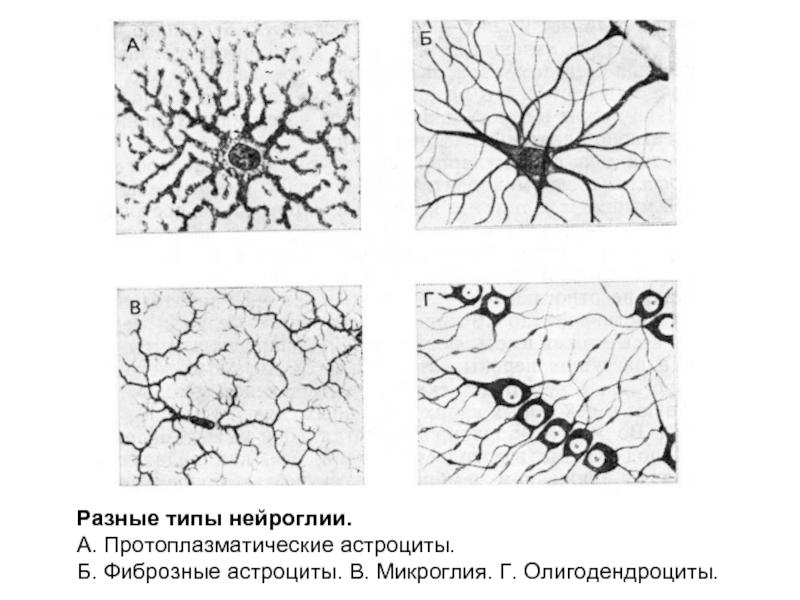
Слайд 15Разные типы нейроглии.
А. Протоплазматические астроциты.
Б. Фиброзные астроциты. В. Микроглия. Г.
Олигодендроциты.
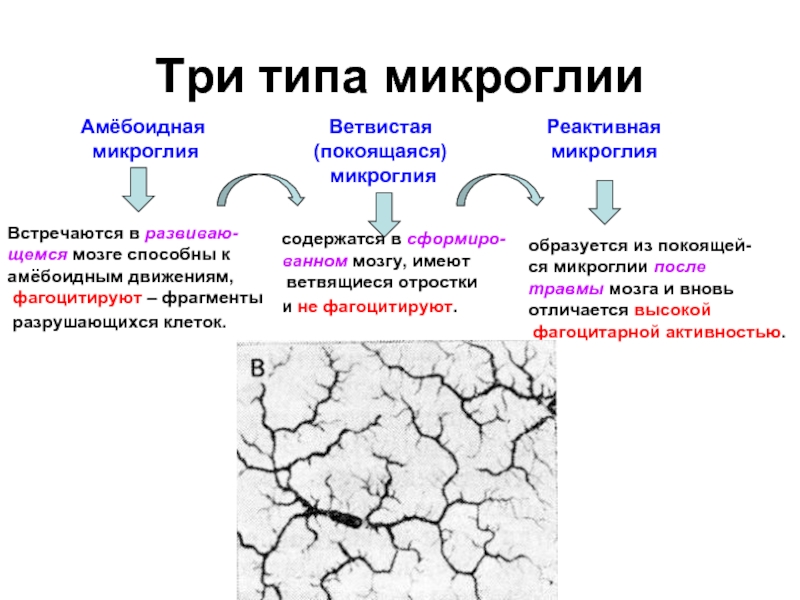
Слайд 18Три типа микроглии
Амёбоидная
микроглия
Ветвистая
(покоящаяся)
микроглия
Реактивная
микроглия
Встречаются в развиваю-
щемся
мозге способны к
амёбоидным движениям,
фагоцитируют – фрагменты
разрушающихся клеток.
содержатся в сформиро-
ванном мозгу, имеют
ветвящиеся отростки
и не фагоцитируют.
образуется из покоящей-
ся микроглии после
травмы мозга и вновь
отличается высокой
фагоцитарной активностью.
Слайд 19Микроглия в сером веществе головного мозга
1 –капилляры,
2 –ядра нейронов,
3
– микро-глиальные клетки — выполняют роль глиальных макрофагов.
Считают, что у больных СПИДом
микроглиоциты (благодаря своей высокой
подвижности) разносят вирус по ЦНС.

Слайд 20Эпендимная глия
ЛОКАЛИЗАЦИЯ:
выстилает спинномозговой канал и желудочки мозга
в отличие
от других видов эпителия, не имеет базальной мембраны, кератиновых филаментов, нет десмосом, на апикальной поверхности микроворсинки и подвижные реснички (киноцилии)
ФУНКЦИИ:
Продукция и перемещение ликвора
Барьерные свойства (гематоликворный барьер).
Транспортная функция (отростки таницитов передают одни вещества из гипоталамуса в гипофиз и другие – в обратном направлении).

Слайд 21Эпендимальная нейроглия
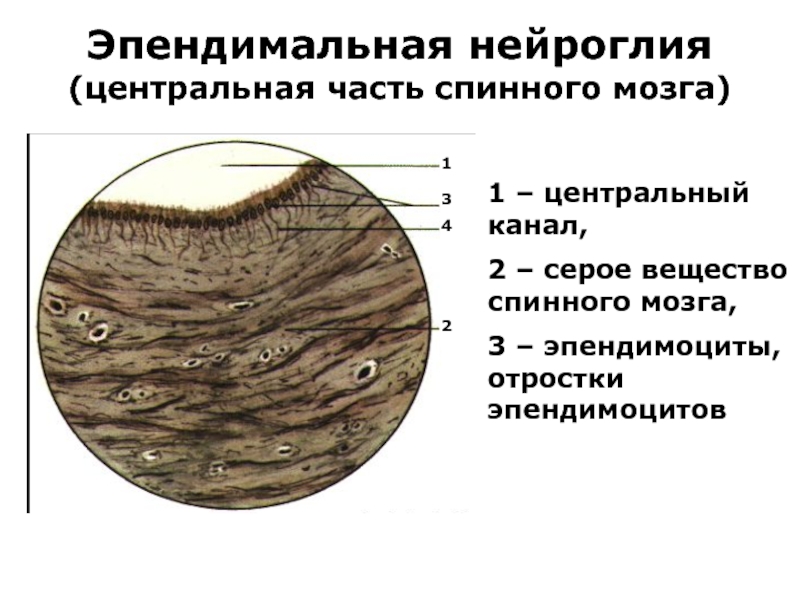
(центральная часть спинного мозга)
1 – центральный канал,
2 – серое
вещество спинного мозга,
3 – эпендимоциты, отростки эпендимоцитов
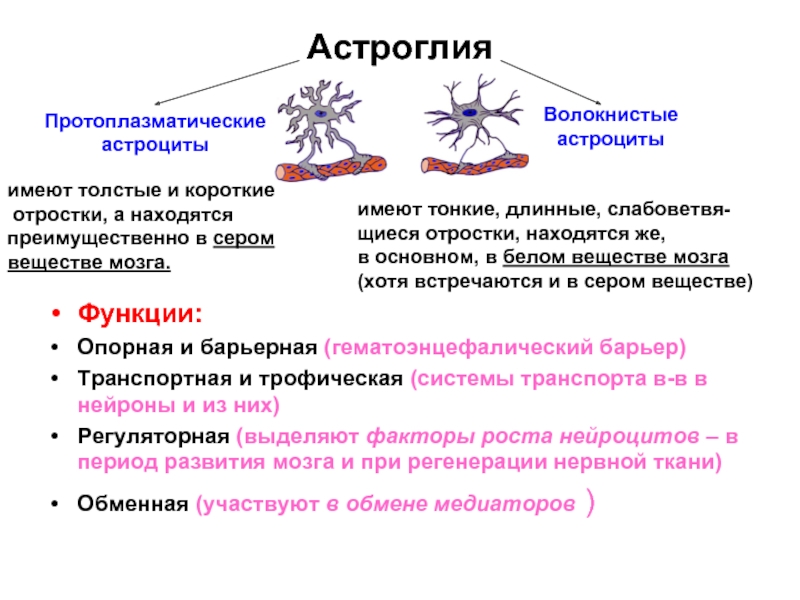
Слайд 22Астроглия
Функции:
Опорная и барьерная (гематоэнцефалический барьер)
Транспортная и трофическая (системы транспорта в-в в
нейроны и из них)
Регуляторная (выделяют факторы роста нейроцитов – в период развития мозга и при регенерации нервной ткани)
Обменная (участвуют в обмене медиаторов )
Протоплазматические
астроциты
Волокнистые
астроциты
имеют толстые и короткие
отростки, а находятся
преимущественно в сером
веществе мозга.
имеют тонкие, длинные, слабоветвя-
щиеся отростки, находятся же,
в основном, в белом веществе мозга
(хотя встречаются и в сером веществе)
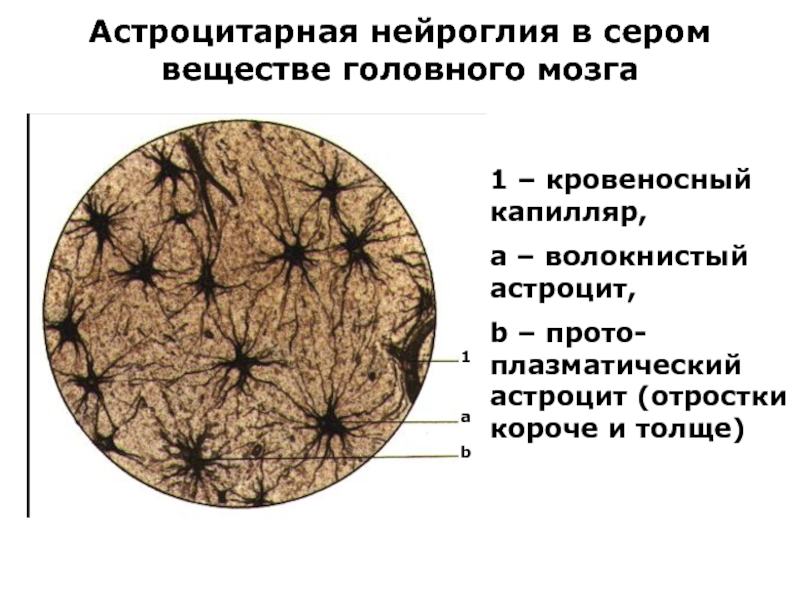
Слайд 23Астроцитарная нейроглия в сером веществе головного мозга
1 – кровеносный капилляр,
a
– волокнистый астроцит,
b – прото-плазматический астроцит (отростки короче и толще)
1
a
b
Слайд 24Олигодендроглия и периферическая нейроглия
Клетки-сателлиты:
окружают тела нейронов (в сером веществе ЦНС и
в нервных ганглиях);
в нервных ганглиях имеют ещё одно название — мантийные глиоциты
Глиоциты нервных волокон:
окружают отростки нейронов (в белом веществе ЦНС и в периферических нервах), образуя нервные волокна;
в периферической нервной системе имеют ещё два названия леммоциты, или
шванновские клетки .
ФУНКЦИИ: трофическая, барьерная и электроизоляционная.

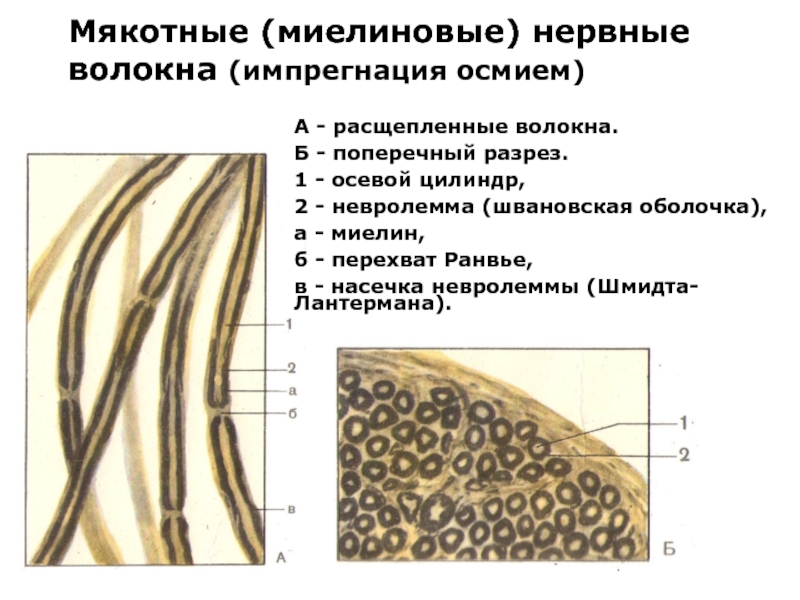
Слайд 28Мякотные (миелиновые) нервные волокна (импрегнация осмием)
А — расщепленные волокна.
Б — поперечный
разрез.
1 — осевой цилиндр,
2 — невролемма (швановская оболочка),
а — миелин,
б — перехват Ранвье,
в — насечка невролеммы (Шмидта-Лантермана).
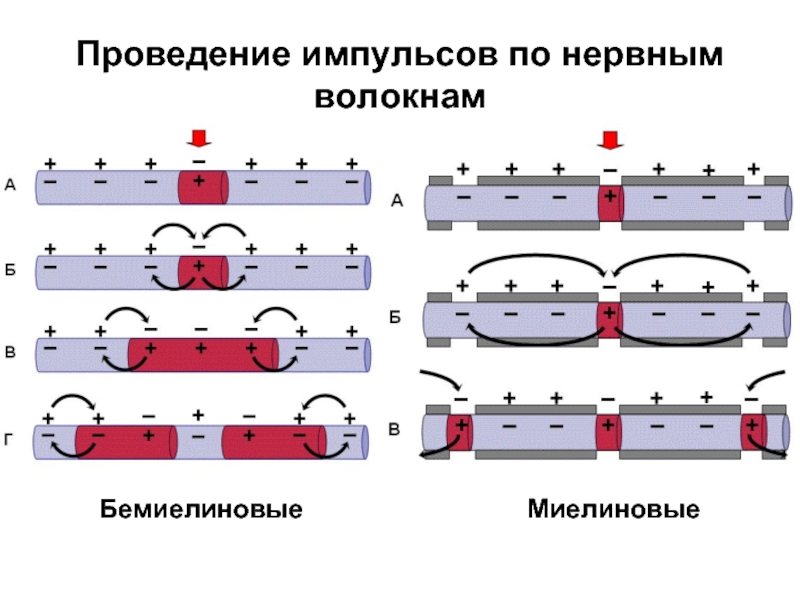
Слайд 32Проведение импульсов по нервным волокнам
Бемиелиновые
Миелиновые
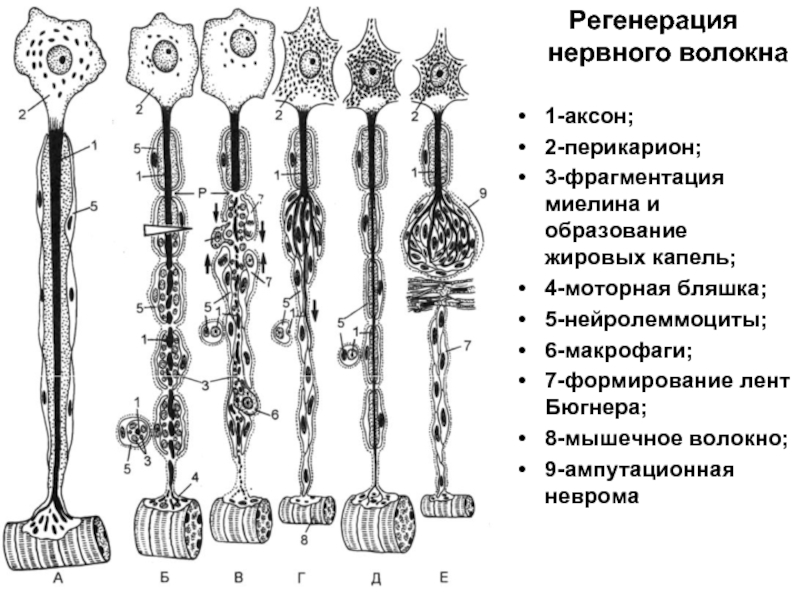
Слайд 33Регенерация нервного волокна
1-аксон;
2-перикарион;
3-фрагментация миелина и образование жировых капель;
4-моторная бляшка;
5-нейролеммоциты;
6-макрофаги;
7-формирование лент Бюгнера;
8-мышечное
волокно;
9-ампутационная неврома
Слайд 34Классификация нервных окончаний
Эффекторные н.о. – это окончания аксонов эффекторных нейронов
(моторные и секреторные)
Рецепторные н. о. — это окончания дендритов
чувствительных нервов.
Окончания, образующие межнейронные синапсы (аксодендритические (между аксоном одного и дендритом другого нейрона); аксосоматические (между аксоном одного и телом другого нейрона); аксоаксональные (между аксонами двух нейронов); соматодендритические синапсы (между телом одного и дендритом другого нейрона). Медиаторы: ацетилхолин, серотонин, норадреналин, ГАМК, дофамин, глицин и многие другие.
Аксовазальные синапсы — это окончания аксонов нейросекреторных нейронов на капиллярах.

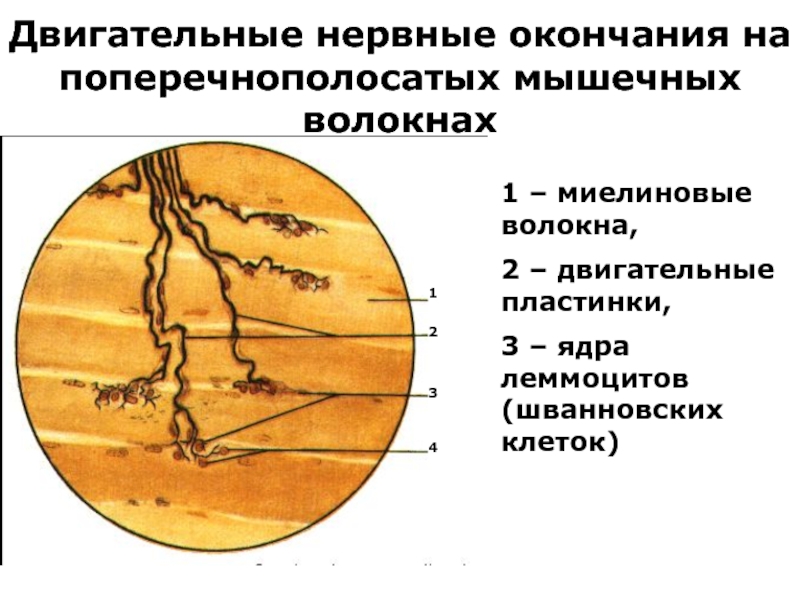
Слайд 36Двигательные нервные окончания на поперечнополосатых мышечных волокнах
1 – миелиновые волокна,
2
– двигательные пластинки,
3 – ядра леммоцитов (шванновских клеток)
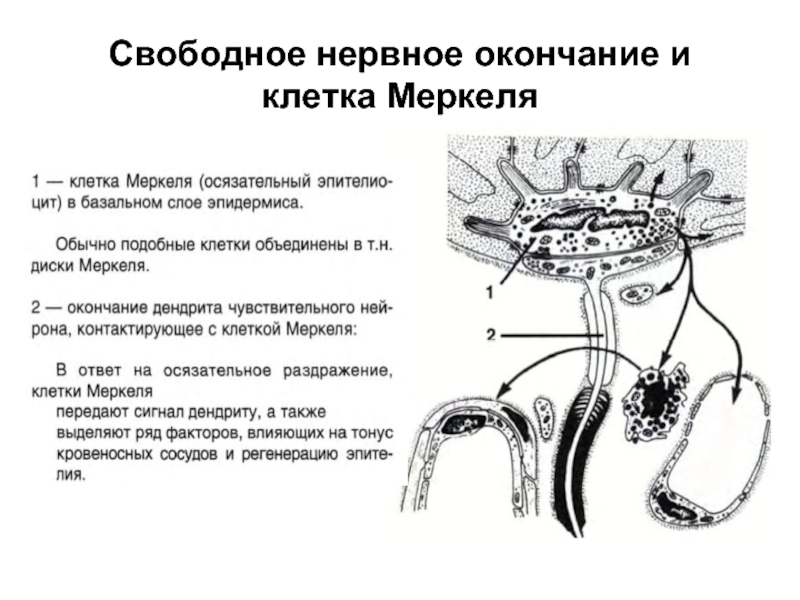
Слайд 38Свободное нервное окончание и клетка Меркеля
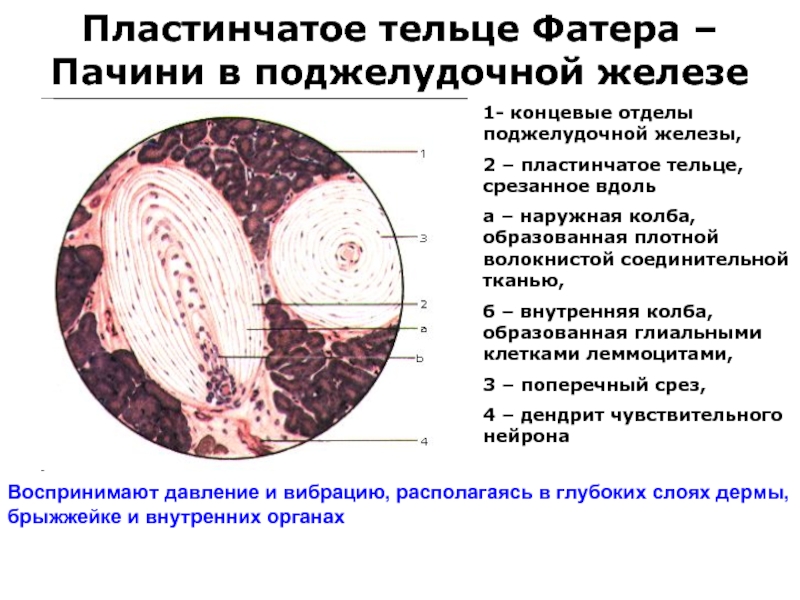
Слайд 39Пластинчатое тельце Фатера – Пачини в поджелудочной железе
1- концевые отделы поджелудочной
железы,
2 – пластинчатое тельце, срезанное вдоль
а – наружная колба, образованная плотной волокнистой соединительной тканью,
б – внутренняя колба, образованная глиальными клетками леммоцитами,
3 – поперечный срез,
4 – дендрит чувствительного нейрона
Воспринимают давление и вибрацию, располагаясь в глубоких слоях дермы,
брыжжейке и внутренних органах
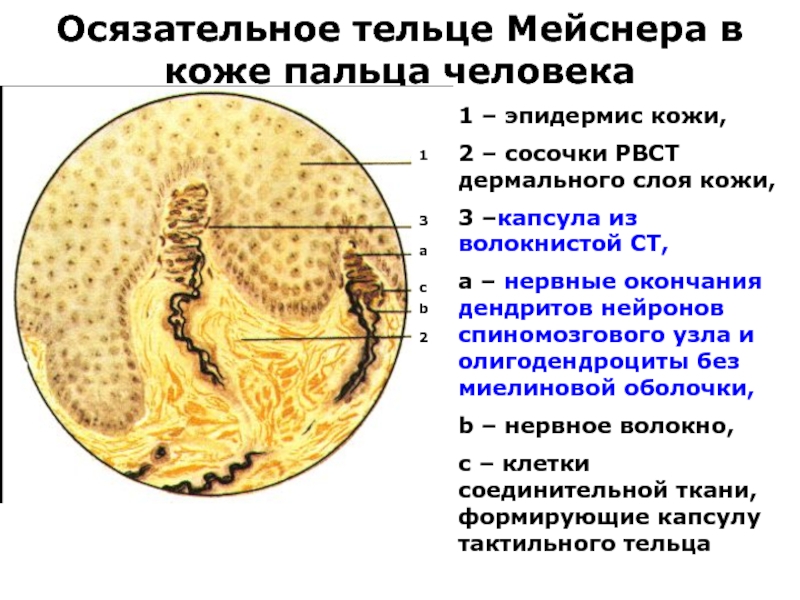
Слайд 40Осязательное тельце Мейснера в коже пальца человека
1 – эпидермис кожи,
2
– сосочки РВСТ дермального слоя кожи,
3 –капсула из волокнистой СТ,
a – нервные окончания дендритов нейронов спиномозгового узла и олигодендроциты без миелиновой оболочки,
b – нервное волокно,
с – клетки соединительной ткани, формирующие капсулу тактильного тельца
Слайд 42Нервно-мышечное веретено
продольный срез скелетной мышцы (г-э)

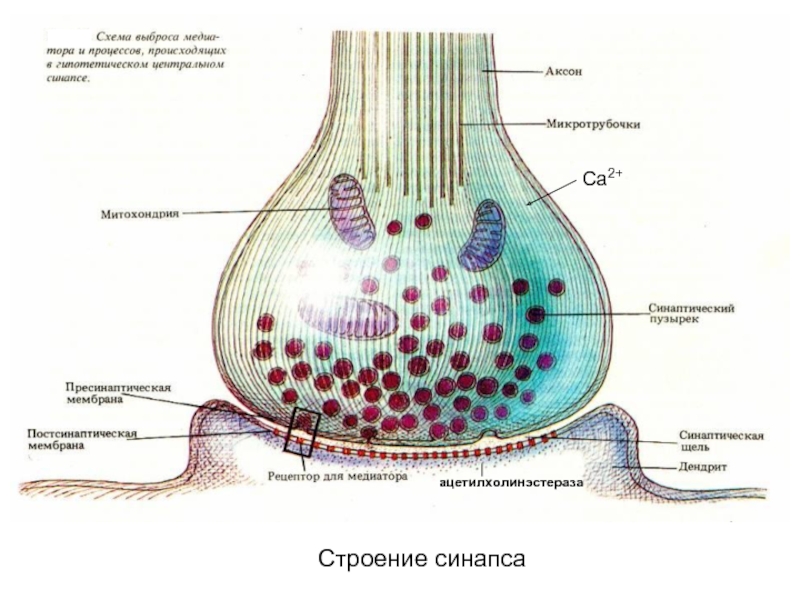
Слайд 44Строение синапса
ацетилхолинэстераза
Са2+
Слайд 46Деполяризующие синапсы необходимы для генерации нервных импульсов, и поэтому потенциалы, возникающие
в таких синапсах, были названы возбуждающими постсинаптическими потенциалами (ВПСП). Деполяризующий ток преимущественно связан со входом ионов Na+.
Ток может быть также связан с открытием каналов либо для выхода катионов К+, либо для входа анионов Cl-. Эти ионные потоки приводят к удержанию мембранного потенциала на уровне покоя или к некоторой гиперполяризаци мембраны. Поскольку эти потенциалы препятствуют деполяризации мембраны и, следовательно, генерации нервных импульсов, их называют тормозными постсинаптическими потенциалами (ТПСП).
Синаптические потенциалы представляют собой градуальные реакции.

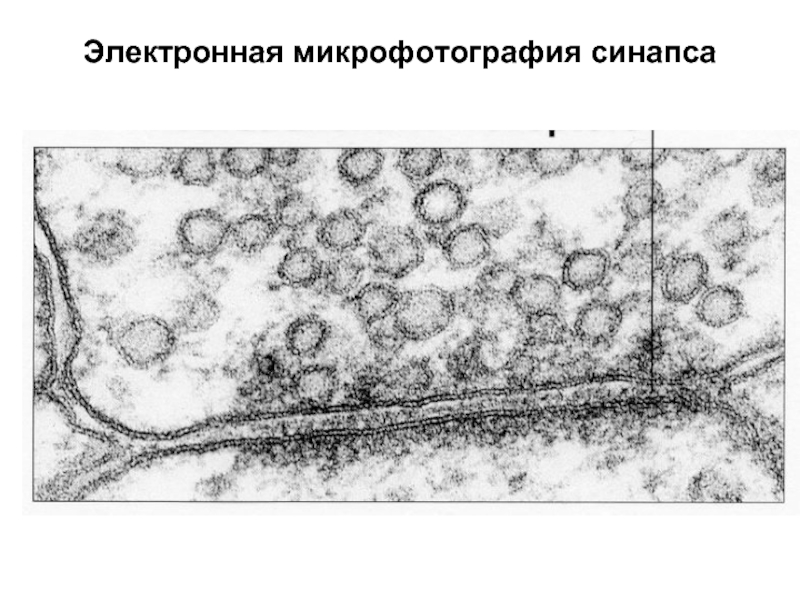
Слайд 47Электронная микрофотография синапса
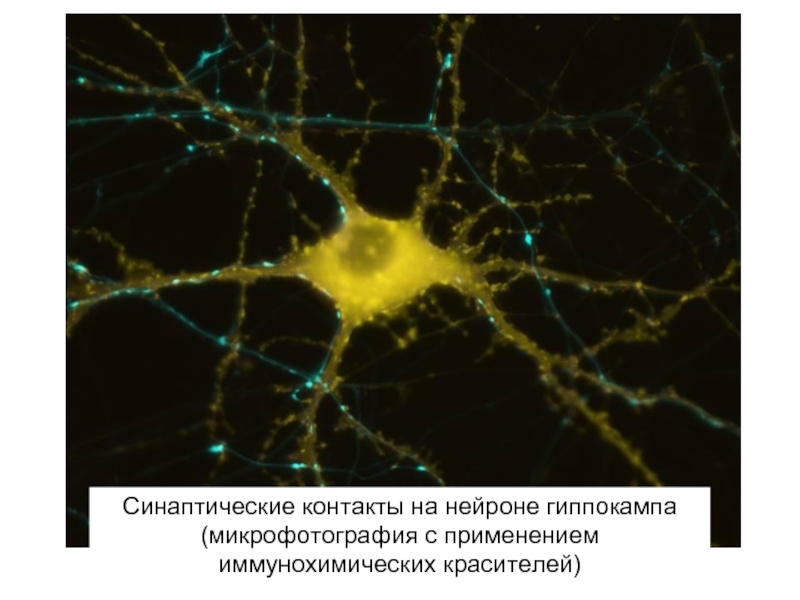
Слайд 48Синаптические контакты на нейроне гиппокампа (микрофотография с применением иммунохимических красителей)
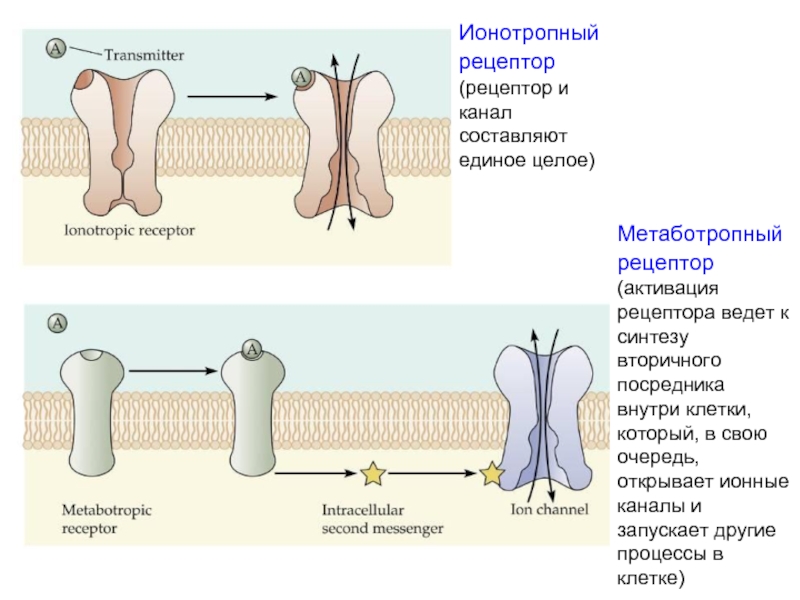
Слайд 49Метаботропный рецептор (активация рецептора ведет к синтезу вторичного посредника внутри клетки,
который, в свою очередь, открывает ионные каналы и запускает другие процессы в клетке)
Ионотропный рецептор (рецептор и канал составляют единое целое)
Слайд 50Ионотропные рецепторы обеспечивают очень быструю реакцию нейрона (порядка нескольких миллисекунд), однако
способны непосредственно влиять лишь на потенциал мембраны нейрона, поэтому участвуют в быстрой передаче сигналов.
Метаботропные рецепторы действуют значительно медленнее (долее 100 мс), однако:
Реакция постсинаптического нейрона более длительная, так как вторичный посредник не сразу разрушается и продолжает действовать в цитоплазме еще некоторое время
Синтез вторичных посредников обеспечивает усиление сигнала (на одну молекулу медиатора синтезируются сотни молекул вторичного посредника)
Вторичные посредники способны влиять одновременно на множество процессов во всем нейроне, в том числе в его ядре.
Соответственно, метаботропные рецепторы обеспечивают нейромодуляцию (настройку «режимов работы» нейронов).

Слайд 51Вторичные посредники (вторичные мессенджеры) передают сигнал внутри нейрона от одной его
части к другой:
— цАМФ (циклический аденозинмонофосфат)
— цГМФ (циклический гуанозинмонофосфат)
— кальций (Ca2+)
окись азота (NO) – [может выступать как ретроградный мессенджер, т.е. передавать сигнал через синапс в обратном направлении]
Примечание: кальций может входить в нейрон через ионотропный канал, но действовать внутри клетки как вторичный посредник.

Слайд 52Агонист – вещество, активирующее рецептор к данному медиатору
Антагонист – вещество,
блокирующее рецептор к данному медиатору или препятствующее действию медиатора
Например, яд кураре – антагонист н-холинорецепторов к ацетилхолину в мышцах, блокирует нервно-мышечную передачу и вызывает временный паралич

Слайд 53Медиаторы (нейротрансмиттеры) передают сигнал от одного нейрона к другому:
Глутамат
– основной возбудительный медиатор в ЦНС
— ГАМК (гамма-аминомасляная кислота) – основной тормозный медиатор в ЦНС
— Глицин — тормозный медиатор в спинном мозге
— Ацетилхолин – возбудительный медиатор (в т.ч. в нервномышечной передаче) и нейромодулятор (внесинаптическое выделение медиатора)
— Норадреналин — нейромодулятор
— Дофамин — нейромодулятор
— Серотонин — нейромодулятор
— Гистамин — нейромодулятор
— Различные пептиды (десятки разных веществ!) — нейромодуляторы
Медиаторы нервной системы

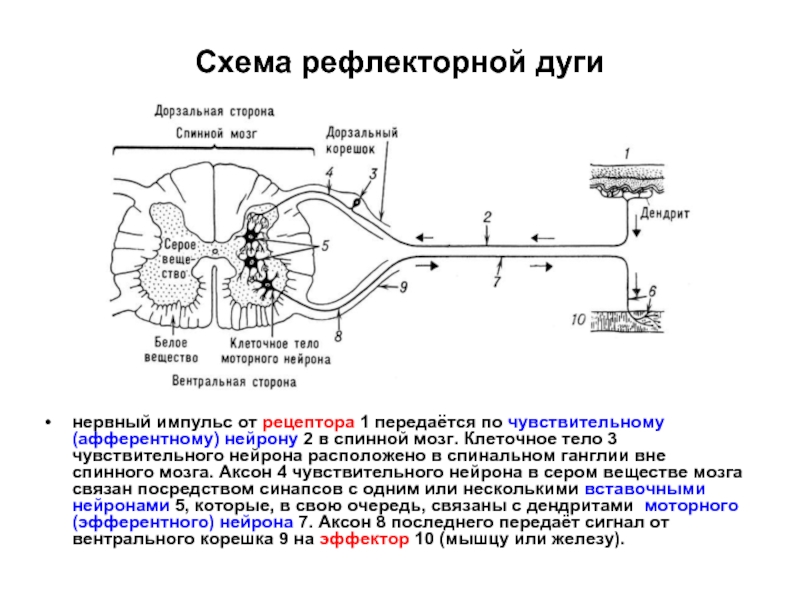
Слайд 54Схема рефлекторной дуги
нервный импульс от рецептора 1 передаётся по чувствительному (афферентному)
нейрону 2 в спинной мозг. Клеточное тело 3 чувствительного нейрона расположено в спинальном ганглии вне спинного мозга. Аксон 4 чувствительного нейрона в сером веществе мозга связан посредством синапсов с одним или несколькими вставочными нейронами 5, которые, в свою очередь, связаны с дендритами моторного (эфферентного) нейрона 7. Аксон 8 последнего передаёт сигнал от вентрального корешка 9 на эффектор 10 (мышцу или железу).