Никита Гудимчук, Павел Захаров, Евгений Ульянов, Фазоил Атауллаханов
«Природа» №10, 2015
Об авторах
|
Никита Борисович Гудимчук — кандидат физико-математических наук, старший научный сотрудник Центра теоретических проблем физико-химической фармакологии РАН и Детского центра гематологии, онкологии и иммунологии им. Дмитрия Рогачева. Область научных интересов — теоретическое и экспериментальное исследование механизмов деления клетки и динамики микротрубочек. |
|
Павел Николаевич Захаров — младший научный сотрудник лаборатории биофизики Детского центра гематологии, онкологии и иммунологии. Занимается математическим моделированием митотического деления клетки. |
|
Евгений Владимирович Ульянов — аспирант физического факультета Московского государственного университета им. М. В. Ломоносова. Область научных исследований — компьютерное моделирование динамики микротрубочек. |
|
Фазоил Иноятович Атауллаханов — доктор биологических наук, профессор МГУ, директор Центра теоретических проблем физико-химической фармакологии, заведующий лабораторией биофизики Детского центра гематологии, онкологии и иммунологии. Научные интересы — клеточная биология, нелинейная динамика и самоорганизация в биологических системах. |
Микротрубочки — один из трех основных типов белковых нитей клетки. Вместе с актиновыми и промежуточными филаментами они образуют клеточный каркас — цитоскелет. Благодаря своим уникальным механическим свойствам микротрубочки выполняют целый ряд ключевых функций на всех этапах жизни клетки, в том числе помогают организовать ее содержимое и служат «рельсами» для направленного транспорта внутриклеточных «грузов» — везикул и органелл. Микротрубочки — динамические структуры, они постоянно меняют свою длину за счет роста или укорачивания. Такое поведение, называемое динамической нестабильностью, существенно влияет на различные внутриклеточные процессы. Например, если клетка выпячивает часть цитоплазмы во время амебоидного движения, микротрубочки быстро заполняют новый объем, повышая в нем интенсивность внутриклеточного транспорта. Часть этих филаментов избирательно стабилизируется, тем самым задавая направление, вдоль которого перемещение «грузов» происходит более регулярно. Вдоль выделенной линии активизируются внутриклеточные процессы, а значит, создаются условия для возникновения у клетки полярности. Главенствующую роль динамика микротрубочек играет во время клеточного деления. Их способность менять длину интенсивно исследуется уже более 30 лет, однако механизмы, лежащие в основе этого феномена, все еще плохо изучены.
Строение и свойства микротрубочек
Микротрубочки — это линейные полимеры. Они построены из димеров белка тубулина, которые образуют 13 цепей — протофиламентов (рис. 1). Каждый из них по бокам связан с двумя другими, и вся конструкция замкнута в цилиндр диаметром 25 нм. Такое строение обеспечивает микротрубочке прочность и большую изгибную жесткость: она может оставаться почти абсолютно прямой в масштабе клетки. Чтобы представить, насколько микротрубочка сложно сгибаема, мысленно увеличим ее до размеров стержня диаметром спагетти (около 2 мм). Такая «спица» не прогибалась бы, будь она длиной даже в сотни метров (высота современных небоскребов)! Жесткость позволяет микротрубочкам выполнять роль длинных прямых направляющих, которые организуют движение органелл внутри клетки. Остальные элементы цитоскелета (актиновые и промежуточные филаменты) существенно более гибкие, поэтому, как правило, используются клеткой в других целях.
Димер тубулина, из которого строится микротрубочка, состоит из мономеров двух типов. Внутри каждого протофиламента α-мономеры одного димера соединяются с β-мономерами соседнего. Поэтому по всей длине микротрубочки, содержащей десятки и сотни тысяч димеров тубулина, все они ориентированы одинаково. Тот конец микротрубочки, к которому обращены α-тубулины, называется минус-концом, а противоположный — плюс-концом. Благодаря такому упорядоченному расположению димеров микротрубочка имеет полярность, что обеспечивает направленность транспорта. Моторные белки, которые участвуют в перемещении «грузов» из одной части клетки в другую, «шагают» по микротрубочке, перетаскивая свою «ношу» за собой, как правило, только в одном направлении. Например, белок динеин двигает органеллы к минус-концу микротрубочки, а кинезин — к плюс-концу. Часто микротрубочки расположены в клетке радиально, а их плюс-концы направлены к ее периферии. Таким образом, кинезины осуществляют транспортировку из центра к внешней мембране, а динеины — от нее внутрь клетки. Поразительно, но в отростках аксонов везикулы и органеллы могут направленно передвигаться по микротрубочкам на расстояния в сотни микрометров и больше.
Динамическая нестабильность: в клетках и в пробирке
От обычных биополимеров микротрубочки отличаются не только механическими свойствами, но и уникальным динамическим поведением (рис. 2). Обычный полимер растет монотонно до тех пор, пока скорость присоединения новых субъединиц из раствора не сравняется со скоростью отделения уже прикрепленных. Полимеризация же микротрубочки носит колебательный характер. Ее длина попеременно то увеличивается, то уменьшается при фиксированной концентрации димеров тубулина в растворе. В одних и тех же условиях сосуществуют растущие и укорачивающиеся микротрубочки. Переходы от стадии роста к укорочению называют катастрофами, а обратные — спасениями. Впервые такое поведение — динамическую нестабильность — обнаружили Т. Митчисон (T. Mitchison) и М. Киршнер (M. Kirschner) около 30 лет назад [1].
Динамическая нестабильность микротрубочек особенно важна во время митоза. Из них строится специальный аппарат для разделения клетки — веретено деления. Оно центрируется благодаря микротрубочкам, которые отталкиваются от клеточной мембраны. Далее, удлиняясь и укорачиваясь, они «обыскивают» пространство клетки в поисках хромосом. Отыскав их и закрепившись за них своими концами, микротрубочки развивают тянущие и толкающие силы, перемещая хромосомы к экватору клетки. Четко выстроив на нем генетический материал и тем самым обеспечив готовность клетки к разделению, микротрубочки растаскивают хромосомы к клеточным полюсам. Все это происходит благодаря динамической нестабильности микротрубочек. Незаменимая роль динамики микротрубочек в митозе привела к разработке лекарств от онкологических заболеваний. Так, например, низкомолекулярное вещество таксол — известный противоопухолевый препарат, стабилизирующий микротрубочки, а значит, останавливающий деление раковых клеток.
Нестабильность микротрубочек проявляется не только в клетках, но и в пробирке — в растворе образующего их белка. Следовательно, для проявления ими этого свойства не требуется ничего, кроме тубулина. Он присоединяется из раствора к концу микротрубочки во время фазы ее роста или, наоборот, отделяется и уходит обратно в раствор во время стадии укорачивания. Тем не менее, другие клеточные белки могут влиять на параметры динамической нестабильности, например, ускорять рост микротрубочек в клетках, менять (увеличивать или уменьшать) частоты катастроф и спасений. Известно, что в пробирке скорость роста микротрубочек и эти частоты многократно ниже, чем в клетках при той же концентрации тубулина.
Модель ГТФ-«шапочки»
Почему микротрубочки, в отличие от других биополимеров, динамически нестабильны? Рост микротрубочки, как сказано, происходит благодаря присоединению к ее концу димеров тубулина. Каждый мономер этого белка связан с молекулой гуанозинтрифосфата (ГТФ). Однако вскоре после присоединения тубулина к микротрубочке молекула ГТФ, связанная с β-субъединицей, гидролизуется до гуанозиндифосфата (ГДФ). ГТФ-димеры тубулина в составе протофиламента стремятся вытянуться, образовать линейную структуру, а ГДФ-димеры — изогнуться в рожок с радиусом кривизны около 20 нм. За счет постоянного присоединения ГТФ-димеров микротрубочка удлиняется, а на ее конце формируется «пояс» из молекул, еще не успевших гидролизовать ГТФ. Пытаясь выпрямиться, этот слой — ГТФ-«колпачок» (или «шапочка») — не дает выгнуться наружу нижележащим ГДФ-димерам и таким образом предохраняет растущий конец микротрубочки от разборки. Считается, что микротрубочка устойчиво растет и защищена от катастрофы, пока на ее конце есть ГТФ-«шапочка». Исчезновение последней в результате гидролиза или случайного отделения ГТФ-димеров тубулина переводит микротрубочку в фазу укорочения.
Модель ГТФ-«шапочки» появилась практически сразу после открытия динамической нестабильности и покорила исследователей своей простотой и элегантностью. Получено уже довольно много экспериментальных фактов, подтверждающих эту модель. Один из классических опытов, показывающих, что на конце микротрубочки есть некая стабилизирующая структура, заключается в следующем. Растущую микротрубочку перерезают микроиглой или сфокусированным пучком ультрафиолетового света [2, 3]. Плюс-конец с отрезанной стороны немедленно начинает разбираться. Интересно, что минус-конец со стороны разреза обычно не разбирается, а продолжает расти. Р. Никлас (R. Nicklas) делал похожий опыт, но разрезал с помощью микроиглы микротрубочку в митотическом веретене внутри клетки [4]. Как и в предыдущем случае, микротрубочка тут же разбиралась со стороны разреза на плюс-конце и оставалась стабильной на минус-конце. Поведение последнего до сих пор остается загадкой, но результаты этих экспериментов сочли сильным доводом, подтверждающим наличие на растущем плюс-конце микротрубочки стабилизирующей ГТФ-«шапочки».
Другой важный аргумент в пользу этой модели появился, когда создали химически модифицированный ГТФ — очень похожий на свой прообраз, но практически неспособный к гидролизу. Когда в растворе плавают только такие молекулы, микротрубочки хорошо растут, но никогда не испытывают катастрофы [5]. Такое поведение подтверждает гипотезу о ГТФ-«шапочке»: ее слабогидролизуемый аналог никак не меняется со временем, а значит, не позволяет микротрубочке разбираться.
Косвенных доказательств существования ГТФ-«шапочки» много, однако ее до сих пор не удалось напрямую увидеть (хотя такие попытки предпринимались). По крайней мере, оценили размер минимальной структуры из слабогидролизуемого аналога ГТФ, которой достаточно, чтобы стабилизировать рост микротрубочки. Защитить ее от разборки, как оказалось, может «шапочка» всего в один слой димеров (при этом реально она может быть и толще). Наглядный способ оценить количество ГТФ-димеров на конце растущей микротрубочки — добавить белок с флуоресцентной меткой, который их распознает. Так называемый плюс-концевой белок EB1 in vitro светится на расстоянии порядка сотни слоев тубулина, причем интенсивность флуоресценции падает от конца к телу микротрубочки. Если этот белок действительно предпочитает связываться именно с ГТФ-димерами, то подобное распределение свечения указывает на то, что ГТФ-«шапочка» может быть значительно больше одного слоя. Примечательно, что белок ЕВ1 ярко окрашивает концы растущих микротрубочек, но начинает гаснуть за несколько секунд перед переходом филамента к катастрофе, как будто отражая постепенное исчезновение стабилизирующей ГТФ-«шапочки» [6]. Измеренная интенсивность флуоресценции белка EB1 на концах микротрубочек в живых клетках также свидетельствует в пользу большой (существенно толще одного слоя тубулинов) ГТФ-«шапочки» [7]. Кроме мечения микротрубочек белком EB1, «шапочку» также визуализировали в клетках с помощью специальных антител, узнающих ГТФ-тубулин [8]. Интересно, что они связывались не только с концами микротрубочек, но и образовывали «островки» на остальной поверхности.
Микротрубочки стареют?
Модель ГТФ-«шапочки» привлекла внимание исследователей прежде всего потому, что позволила объяснить, почему микротрубочка может устойчиво расти и укорачиваться и почему между этими фазами возможны переходы — катастрофы и спасения.
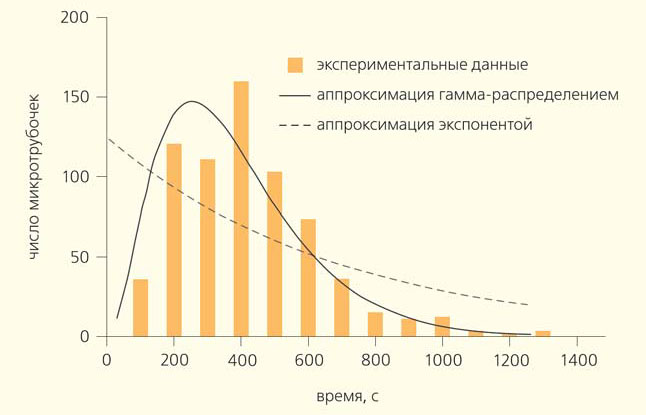
В 1995 г. Д. Одде (D. Odde) с соавторами провел простой, но важный эксперимент [9]. Они наблюдали за ростом микротрубочек в пробирке и решили построить распределение их длин. Оно предполагалось экспоненциальным, но оказалось, что у него есть пик (рис. 3). Значит, в начале роста микротрубочки имеют очень маленькую вероятность испытать катастрофу, а дальше, по мере их роста, эта вероятность повышается. Если пересчитать распределение длин микротрубочек в частоты катастроф, то получится возрастающая зависимость частоты катастроф от времени. Этот эффект назвали «старением» микротрубочек — они как будто «портятся» со временем. Иначе говоря, «молодые» микротрубочки могут расти стабильно, а «старые» уже более склонны к разборке. Необычное распределение времен жизни микротрубочек хорошо аппроксимируется гамма-распределением, которое характеризует процессы с фиксированным количеством последовательных шагов. Поэтому возникла идея, что лучше всего результаты проведенного эксперимента описывает теория, согласно которой катастрофа микротрубочки происходит за три последовательных стадии, когда в ней накопились определенные дефекты неизвестной природы [10]. Эта гипотеза, исходно достаточно сомнительная, тем не менее существенно подогрела интерес к исследованию динамики микротрубочек на уровне отдельных димеров тубулина.
Чего пока не может эксперимент и как помогает теория?
Обнаруженный феномен «старения» микротрубочек показал, что общепринятая, ставшая классической, модель ГТФ-«шапочки» — некоторое упрощение. Действительно, она только постулирует, что микротрубочка испытывает катастрофу, когда теряет свой стабилизирующий «колпачок», но не объясняет, как и почему это происходит, а также из-за чего же вообще микротрубочка может «стареть». Что за таинственные дефекты накапливаются внутри «стареющей» микротрубочки, приводя ее к катастрофе? Сколько их и в какой последовательности они должны проявляться? Может быть, речь идет о гидролизе отдельных молекул ГТФ внутри «шапочки» или о каком-то другом процессе, зависящем от не установленных пока событий совсем иной природы?
Естественно, исследователи хотели бы как можно тщательнее разглядеть «живые» микротрубочки, чтобы ответить на эти вопросы. Однако современный экспериментальный арсенал не позволяет это сделать. Мы можем или увидеть замороженную (обездвиженную) микротрубочку с нанометровым разрешением, например, с помощью электронного микроскопа, или проследить динамику микротрубочки со скоростью сотни кадров в секунду под оптическим микроскопом. К сожалению, невозможно получить соответствующие данные одновременно, чтобы четко их соотнести. Во многом по вине таких ограничений современной науке неизвестно, каков точный размер ГТФ-«шапочки» и как он меняется со временем, а также какую форму имеют концы микротрубочек и как она определяет их динамику.
На помощь экспериментам приходят теоретические методы исследования, в частности компьютерное моделирование. Оно может воссоздать микротрубочку с очень высоким пространственно-временным разрешением, правда, ценой неизбежных идеализаций и упрощений, адекватность которых нужно тщательно проверять (сравнивая результаты модельного и настоящего экспериментов). Идеальная компьютерная модель должна описывать все имеющиеся экспериментальные данные. Тогда на ее основе можно будет изучить механизмы наблюдаемого поведения микротрубочек и предсказать принцип действия белков, влияющих на динамику этих филаментов в клетках. Также станет возможным подбор химических соединений для управления поведением микротрубочек в медицинских целях.
На сегодняшний день создано множество моделей микротрубочек — от очень простых до весьма сложных. Самыми лучшими оказались наиболее детальные модели — молекулярные, которые учитывают, что микротрубочка состоит из многих протофиламентов и что ее структура дискретна (совокупность отдельных субъединиц — тубулинов). Первые такие модели стали появляться почти сразу после обнаружения динамической нестабильности в 1984 г. Работая с ансамблем взаимодействующих тубулинов, они воссоздают поведение микротрубочки как целого. Со времен первых молекулярных моделей накопилось много новых экспериментальных данных о микротрубочках. С тех пор уточнили их строение, измерили новые зависимости характеристик роста и укорочения от различных параметров, изучили поведение этих филаментов после разбавления тубулина, оценили размер ГТФ-«шапочки», открыли способность концов микротрубочек развивать тянущие и толкающие силы [11–19]. Это позволяло корректировать расчеты и все точнее задавать параметры взаимодействия тубулинов. Однако росли и требования к моделям, поскольку они должны непротиворечиво описывать весь набор имеющихся экспериментальных результатов. Таким образом, способы описания взаимодействия тубулинов совершенствовались и усложнялись. От простых моделей, где субъединицы либо взаимодействуют друг с другом, либо нет, перешли к так называемым молекулярно-механическим (самым современным и наиболее реалистичным). Они рассматривают молекулы тубулина как физические объекты, подчиняющиеся законам механики и движущиеся в поле тепловых соударений и потенциалов притяжения друг к другу [20–22]. В ранних молекулярно-механических расчетах динамики микротрубочек из-за ограниченной производительности компьютеров нельзя было подробно описать взаимодействие тубулинов на основе уравнений движения и с учетом тепловых колебаний. Однако эта цель оставалась очень притягательной для нашей команды, поскольку мы предполагали, что тепловые флуктуации играют существенную роль в динамике микротрубочек.
Новая молекулярно-механическая модель
Ускорения расчетов нам удалось достичь главным образом за счет технологии параллельных вычислений на крупнейшем суперкомпьютере «Ломоносов» (в вычислительном центре МГУ) [23]. Он способен производить 1,7·1015 операций в секунду, что выводит его на первое место в Восточной Европе по производительности.
В рамках нашей новой модели субъединицы тубулина — это шарики, на поверхности которых размещены центры взаимодействий с «соседями» (рис. 4). Рассматриваются два типа взаимодействий — продольные и боковые. Сами шарики могут существовать в двух состояниях, соответствующих ГТФ- и ГДФ-формам. В первом случае центры шариков стремятся выстроиться вдоль прямой, а во втором — вдоль дуги, соответствующей углу 22° (для каждой пары субъединиц). Центры взаимодействия притягиваются на близких расстояниях и перестают «чувствовать» друг друга на больших. Движения шариков описываются уравнениями Ланжевена (следствиями второго закона Ньютона), в которых мы пренебрегаем членами, содержащими ускорения частиц (так как эти слагаемые малы по сравнению с остальными). Субъединицы тубулина, удалившиеся от микротрубочки на расстояние, где они перестают с ней взаимодействовать, исключаются из рассмотрения. Также в систему периодически с некоторой вероятностью вводятся новые ГТФ-тубулины, которые появляются в случайной позиции на конце микротрубочки. Внутри нее они могут с определенной вероятностью подвергаться гидролизу — превращаться в ГДФ-субъединицы, которые тут же хотят расположиться по дуге, т. е. сформировать изогнутый протофиламент. Но последний необязательно сразу изгибается, так как от этого его могут удерживать боковые связи. Полученная таким образом система взаимодействующих тубулинов эволюционирует во времени: микротрубочка растет, испытывает катастрофу, укорачивается, спасается и вновь удлиняется. При этом наша модель хорошо описывает характерные формы концов растущей и укорачивающейся микротрубочек, воспроизводит наблюдаемые в экспериментах зависимости динамических характеристик от концентрации тубулина в растворе, а также феномен «старения» микротрубочек. Итак, с помощью моделирования, исходя из простых и понятных принципов и без каких-либо экзотических допущений, мы получили на экране компьютера виртуальную микротрубочку — объект, обладающий всеми основными свойствами своего реального прототипа. Рассчитав координаты всех субъединиц микротрубочки, мы можем с беспрецедентными разрешением и достоверностью узнать все о каждом элементе модельной микротрубочки в любой момент времени. Остается только проанализировать сложную последовательность событий в жизни микротрубочки и понять, какие из них и как приводят ее к переключению от роста к укорачиванию.
Что же происходит с микротрубочкой перед катастрофой? Сначала мы выяснили, выполняется ли в нашей модели какой-либо из двух ранее предложенных гипотетических сценариев этого события. Согласно одному из них, в структуре микротрубочки по мере ее роста могут возникать и сохраняться дефекты, например «дырки» в стенке, возникающие из-за того, что один из протофиламентов замедляет или прекращает свой рост (рис. 5, а) [10]. В нашей модели нет никаких искусственно вложенных оснований для приостановки роста отдельных протофиламентов. Поэтому такая ситуация практически никогда не реализуется, а следовательно, не может быть объяснением механизма «старения» микротрубочек и возникновения катастроф. Вторая гипотеза гласит, что увеличение склонности микротрубочки испытывать катастрофы («старение») происходит по мере постепенного заострения ее конца (рис. 5, б) [24]. Мы тщательно изучили разброс длин у протофиламентов микротрубочки в нашей модели и выяснили, что он быстро достигает некоей устойчивой формы, после чего микротрубочка остается с этим уровнем заостренности. Даже если искусственно создать конфигурацию микротрубочки с концом, в котором длины отдельных протофиламентов будут сильно различаться, то довольно скоро растущая белковая нить, предоставленная сама себе, достигнет все того же устойчивого уровня заостренности, к которому она обычно стремится. Таким образом, медленное заострение конца растущей микротрубочки тоже не может объяснить феномен ее «старения» в нашей модели. Мы также обратили внимание, что и размер ГТФ-«шапочки» не имеет тенденции постепенно уменьшаться (хотя существенно колеблется во время роста микротрубочки), а значит, он не может быть причиной катастрофы.
Отсутствие явного кандидата на медленный необратимый дестабилизирующий процесс привело нас к мысли, что, возможно, его и вовсе нет. А катастрофа происходит не в результате медленного накопления каких-либо дефектов, а из-за возникновения множества короткоживущих обратимых событий. Они время от времени накапливаются на конце микротрубочки и тогда приводят ее к катастрофе (рис. 5, в). Наиболее вероятное событие, приводящее к дестабилизации микротрубочки, — возникновение изогнутого «рожка» на ее конце. Действительно, если протофиламент отогнулся, то даже в случае присоединения к его концу новых субъединиц тубулина из раствора микротрубочка не становится более стабильной и продолжает укорачиваться. Однако один изогнутый протофиламент может легко обломиться и отделиться от микротрубочки. Поэтому по-настоящему дестабилизирующий эффект будут оказывать только несколько изогнутых протофиламентов, образовавшихся на конце микротрубочки одновременно. Количество непрямых протофиламентов, возникающих незадолго до катастрофы в наших расчетах, подтверждает этот вывод.
Таким образом, компьютерное моделирование позволило пролить свет на механизм возникновения катастроф. Оказалось, что в этом процессе важную роль играет не только число ГТФ-димеров, но и механические конфигурации протофиламентов. Катастрофа — результат единовременного образования множества обратимых короткоживущих событий (изогнутых протофиламентов) на конце микротрубочки. Это дополняет классическую модель ГТФ-«шапочки» недостающими деталями, объясняя, как и почему может происходить катастрофа микротрубочки. Мы надеемся, что компьютерное моделирование со временем позволит ответить и на другие вопросы о динамике этих филаментов. Каков механизм спасения микротрубочек? Почему их плюс- и минус-концы в экспериментах по перерезанию пучком ультрафиолетового света или микроиглой ведут себя по-разному? Как белки-модуляторы и потенциальные лекарства воздействуют на динамику микротрубочек?
Работа выполнена при финансовой поддержке фонда «Династия» (грант для молодых биологов) и гранта Президента РФ для молодых кандидатов наук.
Литература
1. Mitchison T., Kirschner M. Dynamic instability of microtubule growth // Nature. 1984. V. 312. P. 237–242.
2. Walker R. A., Inoué S., Salmon E. D. Asymmetric behavior of severed microtubule ends after ultraviolet-microbeam irradiation of individual microtubules in vitro // J. Cell Biol. 1989. V. 108. P. 931–937.
3. Tran P. T., Walker R. A., Salmon E. D. A metastable intermediate state of microtubule dynamic instability that differs significantly between plus and minus ends // J. Cell Biol. 1997. V. 138. P. 105–117. doi: 10.1083/jcb.138.1.105.
4. Nicklas R. B., Lee G. M., Rieder C. L. et al. Mechanically cut mitotic spindles: clean cuts and stable microtubules // J. Cell Sci. 1989. V. 94. P. 415–423.
5. Hyman A. A., Salser S., Drechsel D. N. et al. Role of GTP hydrolysis in microtubule dynamics: information from a slowly hydrolyzable analogue, GMPCPP // Mol. Biol. Cell. 1992. V. 3. P. 1155–1167. doi: 10.1091/mbc.3.10.1155.
6. Maurer S. P., Fourniol F. J., Bohner G. et al. EBs recognize a nucleotide-dependent structural cap at growing microtubule ends // Cell. 2012. V. 149. P. 371–382. doi: 10.1016/j.cell.2012.02.049.
7. Seetapun D., Castle B. T., McIntyre A. J. et al. Estimating the microtubule GTP cap size in vivo // Curr. Biol. 2012. V. 22. P. 1681–1687. doi: 10.1016/j.cub.2012.06.068.
8. Dimitrov A., Quesnoit M., Moutel S. et al. Detection of GTP-tubulin conformation in vivo reveals a role for GTP remnants in microtubule rescues // Science. 2008. V. 322. P. 1353–1356. doi: 10.1126/science.1165401.
9. Odde D. J., Cassimeris L., Buettner H. M. Kinetics of microtubule catastrophe assessed by probabilistic analysis // Biophys. J. 1995. V. 69. P. 796–802. doi: 10.1016/S0006-3495(95)79953-2.
10. Gardner M. K., Zanic M., Gell C. et al. Depolymerizing kinesins Kip3 and MCAK shape cellular microtubule architecture by differential control of catastrophe // Cell. 2011. V. 147. P. 1092–1103. doi: 10.1016/j.cell.2011.10.037.
11. Mandelkow E. M., Mandelkow E., Milligan R. A. Microtubule dynamics and microtubule caps: a time-resolved cryo-electron microscopy study // J. Cell Biol. 1991. V. 114. P. 977–991.
12. Walker R. A., O’Brien E. T., Pryer N. K. et al. Dynamic instability of individual microtubules analyzed by video light microscopy: rate constants and transition frequencies // J. Cell Biol. 1988. V. 107. P. 1437–1448.
13. Walker R. A., Pryer N. K., Salmon E. D. Dilution of individual microtubules observed in real time in vitro: evidence that cap size is small and independent of elongation rate // J. Cell Biol. 1991. V. 114. P. 73–81.
14. Voter W. A., O’Brien E. T., Erickson H. P. Dilution-induced disassembly of microtubules: relation to dynamic instability and the GTP cap // Cell Motil. Cytoskeleton. 1991. V. 18. P. 55–62.
15. O’Brien E. T., Voter W. A., Erickson H. P. GTP hydrolysis during microtubule assembly // Biochemistry. 1987. V. 26. P. 4148–4156.
16. Drechsel D. N., Kirschner M. W. The minimum GTP cap required to stabilize microtubules // Curr. Biol. 1994. V. 4. P. 1053–1061. doi: 10.1016/S0960-9822(00)00243-8.
17. Caplow M., Shanks J. Evidence that a single monolayer tubulin-GTP cap is both necessary and sufficient to stabilize microtubules // Mol. Biol. Cell. 1996. V. 7. P. 663–675. doi: 10.1091/mbc.7.4.663.
18. Grishchuk E. L., Molodtsov M. I., Ataullakhanov F. I. et al. Force production by disassembling microtubules // Nature. 2005. V. 438. P. 384–388. doi: 10.1038/nature04132.
19. Dogterom M., Yurke B. Measurement of the force-velocity relation for growing microtubules // Science. 1997. V. 278. P. 856–860. doi: 10.1126/science.278.5339.856.
20. Molodtsov M. I., Ermakova E. A., Shnol E. E. et al. A molecular-mechanical model of the microtubule // Biophys. J. 2005. V. 88. P. 3167–3179. doi: 10.1529/biophysj.104.051789.
21. VanBuren V., Cassimeris L., Odde D. J. Mechanochemical model of microtubule structure and self-assembly kinetics // Biophys. J. 2005. V. 89. P. 2911–2926. doi: 10.1529/biophysj.105.060913.
22. Efremov A., Grishchuk E. L., McIntosh J. R. et al. In search of an optimal ring to couple microtubule depolymerization to processive chromosome motions // Proc. Natl. Acad. Sci. USA. 2007. V. 104. P. 19017–19022. doi: 10.1073/pnas.0709524104.
23. Воеводин В. В., Жуматий С. А., Соболев С. И. и др. Практика суперкомпьютера «Ломоносов» // Открытые системы. 2012. Т. 7. С. 36–39.
24. Coombes C. E., Yamamoto A., Kenzie M. R. et al. Evolving tip structures can explain age-dependent microtubule catastrophe // Curr. Biol. 2013. V. 23. P. 1342–1348. doi: 10.1016/j.cub.2013.05.059.
Microtubule and tubulin metrics[1]
Microtubules are polymers of tubulin that form part of the cytoskeleton and provide structure and shape to eukaryotic cells. Microtubules can be as long as 50 micrometres, as wide as 23 to 27 nm[2] and have an inner diameter between 11 and 15 nm.[3] They are formed by the polymerization of a dimer of two globular proteins, alpha and beta tubulin into protofilaments that can then associate laterally to form a hollow tube, the microtubule.[4] The most common form of a microtubule consists of 13 protofilaments in the tubular arrangement.
Microtubules are one of the cytoskeletal filament systems in eukaryotic cells. The microtubule cytoskeleton is involved in the transport of material within cells, carried out by motor proteins that move on the surface of the microtubule.
Microtubules play an important role in a number of cellular processes. They are involved in maintaining the structure of the cell and, together with microfilaments and intermediate filaments, they form the cytoskeleton. They also make up the internal structure of cilia and flagella. They provide platforms for intracellular transport and are involved in a variety of cellular processes, including the movement of secretory vesicles, organelles, and intracellular macromolecular assemblies.[5] They are also involved in cell division (by mitosis and meiosis) and are the main constituents of mitotic spindles, which are used to pull eukaryotic chromosomes apart.
Microtubules are nucleated and organized by microtubule-organizing centres, such as the centrosome found in the center of many animal cells or the basal bodies of cilia and flagella, or the spindle pole bodies found in most fungi.
There are many proteins that bind to microtubules, including the motor proteins dynein and kinesin, microtubule-severing proteins like katanin, and other proteins important for regulating microtubule dynamics.[6] Recently an actin-like protein has been found in the gram-positive bacterium Bacillus thuringiensis, which forms a microtubule-like structure called a nanotubule, involved in plasmid segregation.[7] Other bacterial microtubules have a ring of five protofilaments.
History[edit]
Tubulin and microtubule-mediated processes, like cell locomotion, were seen by early microscopists, like Leeuwenhoek (1677). However, the fibrous nature of flagella and other structures were discovered two centuries later, with improved light microscopes, and confirmed in the 20th century with the electron microscope and biochemical studies.[8]
In vitro assays for microtubule motor proteins such as dynein and kinesin are researched by fluorescently tagging a microtubule and fixing either the microtubule or motor proteins to a microscope slide, then visualizing the slide with video-enhanced microscopy to record the travel of the motor proteins. This allows the movement of the motor proteins along the microtubule or the microtubule moving across the motor proteins.[9] Consequently, some microtubule processes can be determined by kymograph.[10]
Structure[edit]
Cartoon representation of the structure of α(yellow)/β(red)-tubulin heterodimer, GTP and GDP.[11]
In eukaryotes, microtubules are long, hollow cylinders made up of polymerised α- and β-tubulin dimers.[12] The inner space of the hollow microtubule cylinders is referred to as the lumen. The α and β-tubulin subunits are ~50% identical at the amino acid level, and both have a molecular weight of approximately 50 kDa.[13][14]
These α/β-tubulin dimers polymerize end-to-end into linear protofilaments that associate laterally to form a single microtubule, which can then be extended by the addition of more α/β-tubulin dimers. Typically, microtubules are formed by the parallel association of thirteen protofilaments, although microtubules composed of fewer or more protofilaments have been observed in various species [15] as well as in vitro.[16]
Microtubules have a distinct polarity that is critical for their biological function. Tubulin polymerizes end to end, with the β-subunits of one tubulin dimer contacting the α-subunits of the next dimer. Therefore, in a protofilament, one end will have the α-subunits exposed while the other end will have the β-subunits exposed. These ends are designated the (−) and (+) ends, respectively. The protofilaments bundle parallel to one another with the same polarity, so, in a microtubule, there is one end, the (+) end, with only β-subunits exposed, while the other end, the (−) end, has only α-subunits exposed. While microtubule elongation can occur at both the (+) and (−) ends, it is significantly more rapid at the (+) end.[17]
The lateral association of the protofilaments generates a pseudo-helical structure, with one turn of the helix containing 13 tubulin dimers, each from a different protofilament. In the most common «13-3» architecture, the 13th tubulin dimer interacts with the next tubulin dimer with a vertical offset of 3 tubulin monomers due to the helicity of the turn. There are other alternative architectures, such as 11-3, 12-3, 14-3, 15-4, or 16-4, that have been detected at a much lower occurrence.[18] Microtubules can also morph into other forms such as helical filaments, which are observed in protist organisms like foraminifera.[19] There are two distinct types of interactions that can occur between the subunits of lateral protofilaments within the microtubule called the A-type and B-type lattices. In the A-type lattice, the lateral associations of protofilaments occur between adjacent α and β-tubulin subunits (i.e. an α-tubulin subunit from one protofilament interacts with a β-tubulin subunit from an adjacent protofilament). In the B-type lattice, the α and β-tubulin subunits from one protofilament interact with the α and β-tubulin subunits from an adjacent protofilament, respectively. Experimental studies have shown that the B-type lattice is the primary arrangement within microtubules. However, in most microtubules there is a seam in which tubulin subunits interact α-β.[20]
The sequence and exact composition of molecules during microtubule formation can thus be summarised as follows: A β-tubulin connects in the context of a non-existent covalent bond with an α-tubulin, which in connected form are a heterodimer, since they consist of two different polypeptides (β-tubulin and α-tubulin). So after the heterodimers are formed, they join together to form long chains that rise figuratively in one direction (e.g. upwards). These heterodimers, which are connected in a certain direction, form protofilaments. These long chains (protofilaments) now gradually accumulate next to each other so that a tube-like structure is formed, which has a lumen typical of a tube. Accordingly, mostly 13 protofilaments form the outer wall of the microtubules. The heterodimers consist of a positive and negative end, with alpha-tubulin forming the negative end and beta-tubulin the positive end. Due to the fact that the heterodimers are stacked on top of each other, there is always a negative and positive end. Microtubules grow by an addition of heterodimers at the plus end.
Some species of Prosthecobacter also contain microtubules. The structure of these bacterial microtubules is similar to that of eukaryotic microtubules, consisting of a hollow tube of protofilaments assembled from heterodimers of bacterial tubulin A (BtubA) and bacterial tubulin B (BtubB). Both BtubA and BtubB share features of both α- and β-tubulin. Unlike eukaryotic microtubules, bacterial microtubules do not require chaperones to fold.[21] In contrast to the 13 protofilaments of eukaryotic microtubules, bacterial microtubules comprise only five.[22]
Intracellular organization[edit]
Microtubules are part of the cytoskeleton, a structural network within the cell’s cytoplasm. The roles of the microtubule cytoskeleton include mechanical support, organization of the cytoplasm, transport, motility and chromosome segregation. In developing neurons microtubules are known as neurotubules,[23] and they can modulate the dynamics of actin, another component of the cytoskeleton.[24] A microtubule is capable of growing and shrinking in order to generate force, and there are motor proteins that allow organelles and other cellular components to be carried along a microtubule. This combination of roles makes microtubules important for organizing and moving intracellular constituents.
The organization of microtubules in the cell is cell-type specific. In epithelia, the minus-ends of the microtubule polymer are anchored near the site of cell-cell contact and organized along the apical-basal axis. After nucleation, the minus-ends are released and then re-anchored in the periphery by factors such as ninein and PLEKHA7.[25] In this manner, they can facilitate the transport of proteins, vesicles and organelles along the apical-basal axis of the cell. In fibroblasts and other mesenchymal cell-types, microtubules are anchored at the centrosome and radiate with their plus-ends outwards towards the cell periphery (as shown in the first figure). In these cells, the microtubules play important roles in cell migration. Moreover, the polarity of microtubules is acted upon by motor proteins, which organize many components of the cell, including the endoplasmic reticulum and the Golgi apparatus.
Components of the eukaryotic cytoskeleton. Actin filaments are shown in red, microtubules are in green, and the nuclei are in blue. The cytoskeleton provides the cell with an inner framework and enables it to move and change shape.
Microtubule polymerization[edit]
Nucleation[edit]
Nucleation is the event that initiates the formation of microtubules from the tubulin dimer. Microtubules are typically nucleated and organized by organelles called microtubule-organizing centres (MTOCs). Contained within the MTOC is another type of tubulin, γ-tubulin, which is distinct from the α- and β-subunits of the microtubules themselves. The γ-tubulin combines with several other associated proteins to form a lock washer-like structure known as the «γ-tubulin ring complex» (γ-TuRC). This complex acts as a template for α/β-tubulin dimers to begin polymerization; it acts as a cap of the (−) end while microtubule growth continues away from the MTOC in the (+) direction.[26]
The centrosome is the primary MTOC of most cell types. However, microtubules can be nucleated from other sites as well. For example, cilia and flagella have MTOCs at their base termed basal bodies. In addition, work from the Kaverina group at Vanderbilt, as well as others, suggests that the Golgi apparatus can serve as an important platform for the nucleation of microtubules.[27] Because nucleation from the centrosome is inherently symmetrical, Golgi-associated microtubule nucleation may allow the cell to establish asymmetry in the microtubule network. In recent studies, the Vale group at UCSF identified the protein complex augmin as a critical factor for centrosome-dependent, spindle-based microtubule generation. It that has been shown to interact with γ-TuRC and increase microtubule density around the mitotic spindle origin.[28]
Some cell types, such as plant cells, do not contain well defined MTOCs. In these cells, microtubules are nucleated from discrete sites in the cytoplasm. Other cell types, such as trypanosomatid parasites, have a MTOC but it is permanently found at the base of a flagellum. Here, nucleation of microtubules for structural roles and for generation of the mitotic spindle is not from a canonical centriole-like MTOC.
Polymerization[edit]
Following the initial nucleation event, tubulin monomers must be added to the growing polymer. The process of adding or removing monomers depends on the concentration of αβ-tubulin dimers in solution in relation to the critical concentration, which is the steady state concentration of dimers at which there is no longer any net assembly or disassembly at the end of the microtubule. If the dimer concentration is greater than the critical concentration, the microtubule will polymerize and grow. If the concentration is less than the critical concentration, the length of the microtubule will decrease.[29]
Microtubule dynamics[edit]
Dynamic instability[edit]
Animation of the microtubule dynamic instability. Tubulin dimers bound to GTP (red) bind to the growing end of a microtubule and subsequently hydrolyze GTP into GDP (blue).
Dynamic instability refers to the coexistence of assembly and disassembly at the ends of a microtubule. The microtubule can dynamically switch between growing and shrinking phases in this region.[30] Tubulin dimers can bind two molecules of GTP, one of which can be hydrolyzed subsequent to assembly. During polymerization, the tubulin dimers are in the GTP-bound state.[12] The GTP bound to α-tubulin is stable and it plays a structural function in this bound state. However, the GTP bound to β-tubulin may be hydrolyzed to GDP shortly after assembly. The assembly properties of GDP-tubulin are different from those of GTP-tubulin, as GDP-tubulin is more prone to depolymerization.[31] A GDP-bound tubulin subunit at the tip of a microtubule will tend to fall off, although a GDP-bound tubulin in the middle of a microtubule cannot spontaneously pop out of the polymer. Since tubulin adds onto the end of the microtubule in the GTP-bound state, a cap of GTP-bound tubulin is proposed to exist at the tip of the microtubule, protecting it from disassembly. When hydrolysis catches up to the tip of the microtubule, it begins a rapid depolymerization and shrinkage. This switch from growth to shrinking is called a catastrophe. GTP-bound tubulin can begin adding to the tip of the microtubule again, providing a new cap and protecting the microtubule from shrinking. This is referred to as «rescue».[32]
«Search and capture» model[edit]
In 1986, Marc Kirschner and Tim Mitchison proposed that microtubules use their dynamic properties of growth and shrinkage at their plus ends to probe the three dimensional space of the cell. Plus ends that encounter kinetochores or sites of polarity become captured and no longer display growth or shrinkage. In contrast to normal dynamic microtubules, which have a half-life of 5–10 minutes, the captured microtubules can last for hours. This idea is commonly known as the «search and capture» model.[33] Indeed, work since then has largely validated this idea. At the kinetochore, a variety of complexes have been shown to capture microtubule (+)-ends.[34] Moreover, a (+)-end capping activity for interphase microtubules has also been described.[35] This later activity is mediated by formins,[36] the adenomatous polyposis coli protein, and EB1,[37] a protein that tracks along the growing plus ends of microtubules.
Regulation of microtubule dynamics[edit]
Post-translational modifications[edit]
Image of a fibroblast cell containing fluorescently labeled actin (red) and microtubules (green).
Although most microtubules have a half-life of 5–10 minutes, certain microtubules can remain stable for hours.[35] These stabilized microtubules accumulate post-translational modifications on their tubulin subunits by the action of microtubule-bound enzymes.[38][39] However, once the microtubule depolymerizes, most of these modifications are rapidly reversed by soluble enzymes. Since most modification reactions are slow while their reverse reactions are rapid, modified tubulin is only detected on long-lived stable microtubules. Most of these modifications occur on the C-terminal region of alpha-tubulin. This region, which is rich in negatively charged glutamate, forms relatively unstructured tails that project out from the microtubule and form contacts with motors. Thus, it is believed that tubulin modifications regulate the interaction of motors with the microtubule. Since these stable modified microtubules are typically oriented towards the site of cell polarity in interphase cells, this subset of modified microtubules provide a specialized route that helps deliver vesicles to these polarized zones. These modifications include:
- Detyrosination: the removal of the C-terminal tyrosine from alpha-tubulin. This reaction exposes a glutamate at the new C-terminus. As a result, microtubules that accumulate this modification are often referred to as Glu-microtubules. Although the tubulin carboxypeptidase has yet to be identified, the tubulin—tyrosine ligase (TTL) is known.[40]
- Delta2: the removal of the last two residues from the C-terminus of alpha-tubulin.[41] Unlike detyrosination, this reaction is thought to be irreversible and has only been documented in neurons.
- Acetylation: the addition of an acetyl group to lysine 40 of alpha-tubulin. This modification occurs on a lysine that is accessible only from the inside of the microtubule, and it remains unclear how enzymes access the lysine residue. The nature of the tubulin acetyltransferase remains controversial, but it has been found that in mammals the major acetyltransferase is ATAT1.[42] however, the reverse reaction is known to be catalyzed by HDAC6.[43]
- Polyglutamylation: the addition of a glutamate polymer (typically 4-6 residues long[44]) to the gamma-carboxyl group of any one of five glutamates found near the end of alpha-tubulin. Enzymes related to TTL add the initial branching glutamate (TTL4,5 and 7), while other enzymes that belong to the same family lengthen the polyglutamate chain (TTL6,11 and 13).[39]
- Polyglycylation: the addition of a glycine polymer (2-10 residues long) to the gamma-carboxyl group of any one of five glutamates found near the end of beta-tubulin. TTL3 and 8 add the initial branching glycine, while TTL10 lengthens the polyglycine chain.[39]
Tubulin is also known to be phosphorylated, ubiquitinated, sumoylated, and palmitoylated.[38]
Tubulin-binding drugs and chemical effects[edit]
A wide variety of drugs are able to bind to tubulin and modify its assembly properties. These drugs can have an effect at intracellular concentrations much lower than that of tubulin. This interference with microtubule dynamics can have the effect of stopping a cell’s cell cycle and can lead to programmed cell death or apoptosis. However, there are data to suggest that interference of microtubule dynamics is insufficient to block the cells undergoing mitosis.[45] These studies have demonstrated that suppression of dynamics occurs at concentrations lower than those needed to block mitosis. Suppression of microtubule dynamics by tubulin mutations or by drug treatment have been shown to inhibit cell migration.[46] Both microtubule stabilizers and destabilizers can suppress microtubule dynamics.
The drugs that can alter microtubule dynamics include:
- The cancer-fighting taxane class of drugs (paclitaxel (taxol) and docetaxel) block dynamic instability by stabilizing GDP-bound tubulin in the microtubule. Thus, even when hydrolysis of GTP reaches the tip of the microtubule, there is no depolymerization and the microtubule does not shrink back.
Taxanes (alone or in combination with platinum derivatives (carboplatine) or gemcitabine) are used against breast and gynecological malignancies, squamous-cell carcinomas (head-and-neck cancers, some lung cancers), etc.
- The epothilones, e.g. Ixabepilone, work in a similar way to the taxanes.
- Vinorelbine, Nocodazole, vincristine, and colchicine have the opposite effect, blocking the polymerization of tubulin into microtubules.
- Eribulin binds to the (+) growing end of the microtubules. Eribulin exerts its anticancer effects by triggering apoptosis of cancer cells following prolonged and irreversible mitotic blockade.
Expression of β3-tubulin has been reported to alter cellular responses to drug-induced suppression of microtubule dynamics. In general the dynamics are normally suppressed by low, subtoxic concentrations of microtubule drugs that also inhibit cell migration. However, incorporating β3-tubulin into microtubules increases the concentration of drug that is needed to suppress dynamics and inhibit cell migration. Thus, tumors that express β3-tubulin are not only resistant to the cytotoxic effects of microtubule targeted drugs, but also to their ability to suppress tumor metastasis.[47] Moreover, expression of β3-tubulin also counteracts the ability of these drugs to inhibit angiogenesis which is normally another important facet of their action.[48]
Microtubule polymers are extremely sensitive to various environmental effects. Very low levels of free calcium can destabilize microtubules and this prevented early researchers from studying the polymer in vitro.[12] Cold temperatures also cause rapid depolymerization of microtubules. In contrast, heavy water promotes microtubule polymer stability.[49]
Proteins that interact with microtubules[edit]
Microtubule-associated proteins (MAPs)[edit]
MAPs have been shown to play a crucial role in the regulation of microtubule dynamics in-vivo. The rates of microtubule polymerization, depolymerization, and catastrophe vary depending on which microtubule-associated proteins (MAPs) are present. The originally identified MAPs from brain tissue can be classified into two groups based on their molecular weight. This first class comprises MAPs with a molecular weight below 55-62 kDa, and are called τ (tau) proteins. In-vitro, tau proteins have been shown to directly bind microtubules, promote nucleation and prevent disassembly, and to induce the formation of parallel arrays.[50] Additionally, tau proteins have also been shown to stabilize microtubules in axons and have been implicated in Alzheimer’s disease.[51] The second class is composed of MAPs with a molecular weight of 200-1000 kDa, of which there are four known types: MAP-1, MAP-2, MAP-3 and MAP-4. MAP-1 proteins consists of a set of three different proteins: A, B and C. The C protein plays an important role in the retrograde transport of vesicles and is also known as cytoplasmic dynein. MAP-2 proteins are located in the dendrites and in the body of neurons, where they bind with other cytoskeletal filaments. The MAP-4 proteins are found in the majority of cells and stabilize microtubules. In addition to MAPs that have a stabilizing effect on microtubule structure, other MAPs can have a destabilizing effect either by cleaving or by inducing depolymerization of microtubules. Three proteins called katanin, spastin, and fidgetin have been observed to regulate the number and length of microtubules via their destabilizing activities. Furthermore, KIAA1211L is predicted to be localized to the microtubules.[52]
MAPs are determinants of different cytoskeletal forms of axons and dendrites, with microtubules being farther apart in the dendrites [53]
Plus-end tracking proteins (+TIPs)[edit]
Plus end tracking proteins are MAP proteins which bind to the tips of growing microtubules and play an important role in regulating microtubule dynamics. For example, +TIPs have been observed to participate in the interactions of microtubules with chromosomes during mitosis. The first MAP to be identified as a +TIP was CLIP170 (cytoplasmic linker protein), which has been shown to play a role in microtubule depolymerization rescue events. Additional examples of +TIPs include EB1, EB2, EB3, p150Glued, Dynamitin, Lis1, CLIP115, CLASP1, and CLASP2.[citation needed]
Motor proteins[edit]
A cytoplasmic dynein motor bound to a microtubule.
A kinesin molecule bound to a microtubule.
Microtubules can act as substrates for motor proteins that are involved in important cellular functions such as vesicle trafficking and cell division. Unlike other microtubule-associated proteins, motor proteins utilize the energy from ATP hydrolysis to generate mechanical work that moves the protein along the substrate. The major motor proteins that interact with microtubules are kinesin, which usually moves toward the (+) end of the microtubule, and dynein, which moves toward the (−) end.
- Dynein is composed of two identical heavy chains, which make up two large globular head domains, and a variable number of intermediate and light chains. Dynein-mediated transport takes place from the (+) end towards the (-) end of the microtubule. ATP hydrolysis occurs in the globular head domains, which share similarities with the AAA+ (ATPase associated with various cellular activities) protein family. ATP hydrolysis in these domains is coupled to movement along the microtubule via the microtubule-binding domains. Dynein transports vesicles and organelles throughout the cytoplasm. In order to do this, dynein molecules bind organelle membranes via a protein complex that contains a number of elements including dynactin.
- Kinesin has a similar structure to dynein. Kinesin is involved in the transport of a variety of intracellular cargoes, including vesicles, organelles, protein complexes, and mRNAs toward the microtubule’s (+) end.[54]
Some viruses (including retroviruses, herpesviruses, parvoviruses, and adenoviruses) that require access to the nucleus to replicate their genomes attach to motor proteins.
Mitosis[edit]
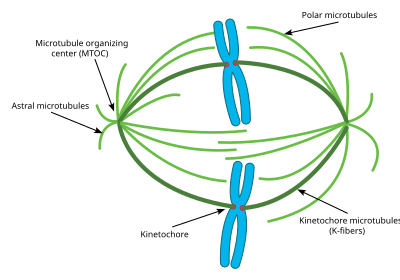
Centrosomes[edit]
A 3D diagram of a centriole. Each circle represents one microtubule. In total there are 27 microtubules organized into 9 bundles of 3.
The centrosome is the main MTOC (microtubule organizing center) of the cell during mitosis. Each centrosome is made up of two cylinders called centrioles, oriented at right angles to each other. The centriole is formed from 9 main microtubules, each having two partial microtubules attached to it. Each centriole is approximately 400 nm long and around 200 nm in circumference.[55]
The centrosome is critical to mitosis as most microtubules involved in the process originate from the centrosome. The minus ends of each microtubule begin at the centrosome, while the plus ends radiate out in all directions. Thus the centrosome is also important in maintaining the polarity of microtubules during mitosis.[56]
Most cells only have one centrosome for most of their cell cycle, however, right before mitosis, the centrosome duplicates, and the cell contains two centrosomes.[57] Some of the microtubules that radiate from the centrosome grow directly away from the sister centrosome. These microtubules are called astral microtubules. With the help of these astral microtubules the centrosomes move away from each other towards opposite sides of the cell. Once there, other types of microtubules necessary for mitosis, including interpolar microtubules and K-fibers can begin to form.[58]
A final important note about the centrosomes and microtubules during mitosis is that while the centrosome is the MTOC for the microtubules necessary for mitosis, research has shown that once the microtubules themselves are formed and in the correct place the centrosomes themselves are not needed for mitosis to occur.[59]
Microtubule subclasses[edit]
This diagram depicts the organization of a typical mitotic spindle found in animal cells. Shown here are the three main types of microtubules during mitosis and how they are oriented in the cell and the mitotic spindle.
Astral microtubules are a subclass of microtubules which only exist during and around mitosis. They originate from the centrosome, but do not interact with the chromosomes, kinetochores, or with the microtubules originating from the other centrosome.[60] Instead their microtubules radiate towards the cell membrane. Once there they interact with specific motor proteins which create force that pull the microtubules, and thus the entire centrosome towards the cell membrane. As stated above, this helps the centrosomes orient themselves away from each other in the cell. However these astral microtubules do not interact with the mitotic spindle itself. Experiments have shown that without these astral microtubules, the mitotic spindle can form, however its orientation in the cell is not always correct and thus mitosis does not occur as effectively.[61] Another key function of the astral microtubules is to aid in cytokinesis. Astral microtubules interact with motor proteins at the cell membrane to pull the spindle and the entire cell apart once the chromosomes have been replicated.
Interpolar/Polar microtubules are a class of microtubules which also radiate out from the centrosome during mitosis. These microtubules radiate towards the mitotic spindle, unlike astral microtubules. Interpolar microtubules are both the most abundant and dynamic subclass of microtubules during mitosis. Around 95 percent of microtubules in the mitotic spindle can be characterized as interpolar. Furthermore, the half life of these microtubules is extremely short as it is less than one minute.[62] Interpolar microtubules that do not attach to the kinetochores can aid in chromosome congregation through lateral interaction with the kinetochores.[63]
K fibers/Kinetochore microtubules are the third important subclass of mitotic microtubules. These microtubules form direct connections with the kinetochores in the mitotic spindle. Each K fiber is composed of 20–40 parallel microtubules, forming a strong tube which is attached at one end to the centrosome and on the other to the kinetochore, located in the center of each chromosome. Since each centrosome has a K fiber connecting to each pair of chromosomes, the chromosomes become tethered in the middle of the mitotic spindle by the K fibers. K fibers have a much longer half life than interpolar microtubules, at between 4 and 8 minutes.[64] During the end of mitoses, the microtubules forming each K fiber begin to disassociate, thus shorting the K fibers. As the K fibers shorten the pair chromosomes are pulled apart right before cytokinesis. Previously, some researchers believed that K fibers form at their minus end originating from the centrosome just like other microtubules, however, new research has pointed to a different mechanism. In this new mechanism, the K fibers are initially stabilized at their plus end by the kinetochores and grow out from there. The minus end of these K fibers eventually connect to an existing Interpolar microtubule and are eventually connected to the centrosome in this way.[65]
Microtubule nuclear in the mitotic spindle[edit]
Most of the microtubules that form the mitotic spindle originate from the centrosome. Originally it was thought that all of these microtubules originated from the centrosome via a method called search and capture, described in more detail in a section above, however new research has shown that there are addition means of microtubule nucleation during mitosis. One of the most important of these additional means of microtubule nucleation is the RAN-GTP pathway. RAN-GTP associates with chromatin during mitosis to create a gradient that allows for local nucleation of microtubules near the chromosomes. Furthermore, a second pathway known as the augmin/HAUS complex (some organisms use the more studied augmin complex, while others such as humans use an analogous complex called HAUS) acts an additional means of microtubule nucleation in the mitotic spindle.[65]
Functions[edit]
Cell migration[edit]
Microtubule plus ends are often localized to particular structures. In polarized interphase cells, microtubules are disproportionately oriented from the MTOC toward the site of polarity, such as the leading edge of migrating fibroblasts. This configuration is thought to help deliver microtubule-bound vesicles from the Golgi to the site of polarity.
Dynamic instability of microtubules is also required for the migration of most mammalian cells that crawl.[66] Dynamic microtubules regulate the levels of key G-proteins such as RhoA[67] and Rac1,[68] which regulate cell contractility and cell spreading. Dynamic microtubules are also required to trigger focal adhesion disassembly, which is necessary for migration.[69] It has been found that microtubules act as “struts” that counteract the contractile forces that are needed for trailing edge retraction during cell movement. When microtubules in the trailing edge of cell are dynamic, they are able to remodel to allow retraction. When dynamics are suppressed, microtubules cannot remodel and, therefore, oppose the contractile forces.[46] The morphology of cells with suppressed microtubule dynamics indicate that cells can extend the front edge (polarized in the direction of movement), but have difficulty retracting their trailing edge.[70] On the other hand, high drug concentrations, or microtubule mutations that depolymerize the microtubules, can restore cell migration but there is a loss of directionality. It can be concluded that microtubules act both to restrain cell movement and to establish directionality.
Cilia and flagella[edit]
Microtubules have a major structural role in eukaryotic cilia and flagella. Cilia and flagella always extend directly from a MTOC, in this case termed the basal body. The action of the dynein motor proteins on the various microtubule strands that run along a cilium or flagellum allows the organelle to bend and generate force for swimming, moving extracellular material, and other roles. Prokaryotes possess tubulin-like proteins including FtsZ. However, prokaryotic flagella are entirely different in structure from eukaryotic flagella and do not contain microtubule-based structures.
Development[edit]
The cytoskeleton formed by microtubules is essential to the morphogenetic process of an organism’s development. For example, a network of polarized microtubules is required within the oocyte of Drosophila melanogaster during its embryogenesis in order to establish the axis of the egg. Signals sent between the follicular cells and the oocyte (such as factors similar to epidermal growth factor) cause the reorganization of the microtubules so that their (-) ends are located in the lower part of the oocyte, polarizing the structure and leading to the appearance of an anterior-posterior axis.[71] This involvement in the body’s architecture is also seen in mammals.[72]
Another area where microtubules are essential is the development of the nervous system in higher vertebrates, where tubulin’s dynamics and those of the associated proteins (such as the microtubule-associated proteins) are finely controlled during the development of the nervous system.[73]
Gene regulation[edit]
The cellular cytoskeleton is a dynamic system that functions on many different levels: In addition to giving the cell a particular form and supporting the transport of vesicles and organelles, it can also influence gene expression. The signal transduction mechanisms involved in this communication are little understood. However, the relationship between the drug-mediated depolymerization of microtubules, and the specific expression of transcription factors has been described, which has provided information on the differential expression of the genes depending on the presence of these factors.[74] This communication between the cytoskeleton and the regulation of the cellular response is also related to the action of growth factors: for example, this relation exists for connective tissue growth factor.[75]
See also[edit]
- Microtentacle
- Orchestrated objective reduction – a hypothesis explaining consciousness
References[edit]
- ^ «Digital Downloads». PurSolutions. Retrieved 2020-02-20.
- ^ Ledbetter MC, Porter KR (1963). «A «microtubule» in plant cell fine structure». Journal of Cell Biology. 19 (1): 239–50. doi:10.1083/jcb.19.1.239. PMC 2106853. PMID 19866635.
- ^ Chalfie M, Thomson JN (1979). «Organization of neuronal microtubules in the nematode Caenorhabditis elegans». Journal of Cell Biology. 82 (1): 278–89. doi:10.1083/jcb.82.1.278. PMC 2110421. PMID 479300.
- ^ Diwan JJ (2006). «Microtubules». Rensselaer Polytechnic Institute. Archived from the original on 2014-02-06. Retrieved 2014-02-24.
- ^ Vale RD (February 2003). «The molecular motor toolbox for intracellular transport». Cell. 112 (4): 467–80. doi:10.1016/S0092-8674(03)00111-9. PMID 12600311. S2CID 15100327.
- ^ Howard J, Hyman AA (February 2007). «Microtubule polymerases and depolymerases». Current Opinion in Cell Biology. 19 (1): 31–5. doi:10.1016/j.ceb.2006.12.009. PMID 17184986.
- ^ Jiang S, Narita A, Popp D, Ghoshdastider U, Lee LJ, Srinivasan R, Balasubramanian MK, Oda T, Koh F, Larsson M, Robinson RC (March 2016). «Novel actin filaments from Bacillus thuringiensis form nanotubules for plasmid DNA segregation». Proceedings of the National Academy of Sciences of the United States of America. 113 (9): E1200-5. Bibcode:2016PNAS..113E1200J. doi:10.1073/pnas.1600129113. PMC 4780641. PMID 26873105.
- ^ Wayne, R. 2009. Plant Cell Biology: From Astronomy to Zoology. Amsterdam: Elsevier/Academic Press, p. 165.
- ^ Cooper GM (2000). «Microtubule Motors and Movements». The Cell: A Molecular Approach. 2nd Edition. Retrieved 2019-03-12.
- ^ Kapoor V, Hirst WG, Hentschel C, Preibisch S, Reber S (March 2019). «MTrack: Automated Detection, Tracking, and Analysis of Dynamic Microtubules». Scientific Reports. 9 (1): 3794. Bibcode:2019NatSR…9.3794K. doi:10.1038/s41598-018-37767-1. PMC 6405942. PMID 30846705.
- ^ Löwe J, Li H, Downing KH, Nogales E (November 2001). «Refined structure of alpha beta-tubulin at 3.5 A resolution». Journal of Molecular Biology. 313 (5): 1045–57. doi:10.1006/jmbi.2001.5077. PMID 11700061.
- ^ a b c Weisenberg RC (September 1972). «Microtubule formation in vitro in solutions containing low calcium concentrations». Science. 177 (4054): 1104–5. Bibcode:1972Sci…177.1104W. doi:10.1126/science.177.4054.1104. PMID 4626639. S2CID 34875893.
- ^ Desai A, Mitchison TJ (1997). «Microtubule polymerization dynamics». Annual Review of Cell and Developmental Biology. 13: 83–117. doi:10.1146/annurev.cellbio.13.1.83. PMID 9442869.
- ^ Desai, A.; Mitchison, T. J. (1997). «Microtubule polymerization dynamics». Annual Review of Cell and Developmental Biology. 13: 83–117. doi:10.1146/annurev.cellbio.13.1.83. ISSN 1081-0706. PMID 9442869.
- ^ Chaaban S, Brouhard GJ (2017). «A microtubule bestiary: structural diversity in tubulin polymers». Molecular Biology of the Cell. 28 (22): 2924–31. doi:10.1091/mbc.E16-05-0271. PMC 5662251. PMID 29084910.
- ^ Chrétien D, Metoz F, Verde F, Karsenti E, Wade RH (June 1992). «Lattice defects in microtubules: protofilament numbers vary within individual microtubules». Journal of Cell Biology. 117 (5): 1031–40. doi:10.1083/jcb.117.5.1031. PMC 2289483. PMID 1577866.
- ^ Walker RA, O’Brien ET, Pryer NK, Soboeiro MF, Voter WA, Erickson HP, Salmon ED (October 1988). «Dynamic instability of individual microtubules analyzed by video light microscopy: rate constants and transition frequencies». The Journal of Cell Biology. 107 (4): 1437–48. CiteSeerX 10.1.1.525.507. doi:10.1083/jcb.107.4.1437. PMC 2115242. PMID 3170635.
- ^ Sui H, Downing KH (August 2010). «Structural basis of interprotofilament interaction and lateral deformation of microtubules». Structure. 18 (8): 1022–31. doi:10.1016/j.str.2010.05.010. PMC 2976607. PMID 20696402.
- ^ Bassen DM, Hou Y, Bowser SS, Banavali NK (August 2016). «Maintenance of electrostatic stabilization in altered tubulin lateral contacts may facilitate formation of helical filaments in foraminifera». Scientific Reports. 6: 31723. Bibcode:2016NatSR…631723B. doi:10.1038/srep31723. PMC 4990898. PMID 27539392.
- ^ Nogales E (2000). «Structural insights into microtubule function». Annual Review of Biochemistry. 69: 277–302. doi:10.1146/annurev.biochem.69.1.277. PMID 10966460.
- ^ Schlieper D, Oliva MA, Andreu JM, Löwe J (June 2005). «Structure of bacterial tubulin BtubA/B: evidence for horizontal gene transfer». Proceedings of the National Academy of Sciences of the United States of America. 102 (26): 9170–5. Bibcode:2005PNAS..102.9170S. doi:10.1073/pnas.0502859102. PMC 1166614. PMID 15967998.
- ^ Pilhofer M, Ladinsky MS, McDowall AW, Petroni G, Jensen GJ (December 2011). «Microtubules in bacteria: Ancient tubulins build a five-protofilament homolog of the eukaryotic cytoskeleton». PLOS Biology. 9 (12): e1001213. doi:10.1371/journal.pbio.1001213. PMC 3232192. PMID 22162949.
- ^ «Medical Definition of Neurotubules». www.merriam-webster.com.
- ^ Zhao B, Meka DP, Scharrenberg R, König T, Schwanke B, Kobler O, Windhorst S, Kreutz MR, Mikhaylova M, Calderon de Anda F (August 2017). «Microtubules Modulate F-actin Dynamics during Neuronal Polarization». Scientific Reports. 7 (1): 9583. Bibcode:2017NatSR…7.9583Z. doi:10.1038/s41598-017-09832-8. PMC 5575062. PMID 28851982.
- ^ Bartolini F, Gundersen GG (October 2006). «Generation of noncentrosomal microtubule arrays». Journal of Cell Science. 119 (Pt 20): 4155–63. doi:10.1242/jcs.03227. PMID 17038542.
- ^ Desai A, Mitchison TJ (1997). «Microtubule polymerization dynamics». Annual Review of Cell and Developmental Biology. 13: 83–117. doi:10.1146/annurev.cellbio.13.1.83. PMID 9442869.
- ^ Vinogradova T, Miller PM, Kaverina I (July 2009). «Microtubule network asymmetry in motile cells: role of Golgi-derived array». Cell Cycle. 8 (14): 2168–74. doi:10.4161/cc.8.14.9074. PMC 3163838. PMID 19556895.
- ^ Uehara R, Nozawa RS, Tomioka A, Petry S, Vale RD, Obuse C, Goshima G (April 2009). «The augmin complex plays a critical role in spindle microtubule generation for mitotic progression and cytokinesis in human cells». Proceedings of the National Academy of Sciences of the United States of America. 106 (17): 6998–7003. Bibcode:2009PNAS..106.6998U. doi:10.1073/pnas.0901587106. PMC 2668966. PMID 19369198.
- ^ Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002). «The Self-Assembly and Dynamic Structure of Cytoskeletal Filaments». Molecular Biology of the Cell (4th ed.). New York: Garland Science.
- ^ Karp G (2005). Cell and Molecular Biology: Concepts and Experiments. USA: John Wiley & Sons. p. 355. ISBN 978-0-471-46580-5.
- ^ Weisenberg RC, Deery WJ, Dickinson PJ (September 1976). «Tubulin-nucleotide interactions during the polymerization and depolymerization of microtubules». Biochemistry. 15 (19): 4248–54. doi:10.1021/bi00664a018. PMID 963034.
- ^ Mitchison T, Kirschner M (1984). «Dynamic instability of microtubule growth». Nature. 312 (5991): 237–42. Bibcode:1984Natur.312..237M. doi:10.1038/312237a0. PMID 6504138. S2CID 30079133.
- ^ Kirschner M, Mitchison T (May 1986). «Beyond self-assembly: from microtubules to morphogenesis». Cell. 45 (3): 329–42. doi:10.1016/0092-8674(86)90318-1. PMID 3516413. S2CID 36994346.
- ^ Cheeseman IM, Desai A (January 2008). «Molecular architecture of the kinetochore-microtubule interface». Nature Reviews. Molecular Cell Biology. 9 (1): 33–46. doi:10.1038/nrm2310. PMID 18097444. S2CID 34121605.
- ^ a b Infante AS, Stein MS, Zhai Y, Borisy GG, Gundersen GG (November 2000). «Detyrosinated (Glu) microtubules are stabilized by an ATP-sensitive plus-end cap». Journal of Cell Science. 113 (22): 3907–19. doi:10.1242/jcs.113.22.3907. PMID 11058078.
- ^ Palazzo AF, Cook TA, Alberts AS, Gundersen GG (August 2001). «mDia mediates Rho-regulated formation and orientation of stable microtubules». Nature Cell Biology. 3 (8): 723–9. doi:10.1038/35087035. PMID 11483957. S2CID 7374170.
- ^ Wen Y, Eng CH, Schmoranzer J, Cabrera-Poch N, Morris EJ, Chen M, Wallar BJ, Alberts AS, Gundersen GG (September 2004). «EB1 and APC bind to mDia to stabilize microtubules downstream of Rho and promote cell migration». Nature Cell Biology. 6 (9): 820–30. doi:10.1038/ncb1160. PMID 15311282. S2CID 29214110.
- ^ a b Janke C, Bulinski JC (November 2011). «Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions». Nature Reviews. Molecular Cell Biology. 12 (12): 773–86. doi:10.1038/nrm3227. PMID 22086369. S2CID 5969290.
- ^ a b c Garnham CP, Roll-Mecak A (July 2012). «The chemical complexity of cellular microtubules: tubulin post-translational modification enzymes and their roles in tuning microtubule functions». Cytoskeleton. 69 (7): 442–63. doi:10.1002/cm.21027. PMC 3459347. PMID 22422711.
- ^ Ersfeld K, Wehland J, Plessmann U, Dodemont H, Gerke V, Weber K (February 1993). «Characterization of the tubulin-tyrosine ligase». The Journal of Cell Biology. 120 (3): 725–32. doi:10.1083/jcb.120.3.725. PMC 2119537. PMID 8093886.
- ^
- ^ Kalebic N, Sorrentino S, Perlas E, Bolasco G, Martinez C, Heppenstall PA (2013-06-10). «αTAT1 is the major α-tubulin acetyltransferase in mice». Nature Communications. 4: 1962. Bibcode:2013NatCo…4.1962K. doi:10.1038/ncomms2962. PMID 23748901.
- ^ Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP (May 2002). «HDAC6 is a microtubule-associated deacetylase». Nature. 417 (6887): 455–8. Bibcode:2002Natur.417..455H. doi:10.1038/417455a. PMID 12024216. S2CID 4373254.
- ^ Audebert S, Desbruyères E, Gruszczynski C, Koulakoff A, Gros F, Denoulet P, Eddé B (June 1993). «Reversible polyglutamylation of alpha- and beta-tubulin and microtubule dynamics in mouse brain neurons». Molecular Biology of the Cell. 4 (6): 615–26. doi:10.1091/mbc.4.6.615. PMC 300968. PMID 8104053.
- ^ Ganguly A, Yang H, Cabral F (November 2010). «Paclitaxel-dependent cell lines reveal a novel drug activity». Molecular Cancer Therapeutics. 9 (11): 2914–23. doi:10.1158/1535-7163.MCT-10-0552. PMC 2978777. PMID 20978163.
- ^ a b Yang H, Ganguly A, Cabral F (October 2010). «Inhibition of cell migration and cell division correlates with distinct effects of microtubule inhibiting drugs». The Journal of Biological Chemistry. 285 (42): 32242–50. doi:10.1074/jbc.M110.160820. PMC 2952225. PMID 20696757.
- ^ Altonsy, Mohammed; Ganguly, Anutosh; Amrein, Matthias; Surmanowicz, Philip; Li, Shu; Lauzon, Gilles (Mar 2020). «Beta3-Tubulin Is Critical for Microtubule Dynamics, Cell Cycle Regulation, and Spontaneous Release of Microvesicles in Human Malignant Melanoma Cells (A375)». International Journal of Molecular Sciences. 21 – via National Library of Medicine.
- ^ Ganguly, Anutosh; Yang, Hailing; Fernando, Gabral (May 2011). «Class III β-Tubulin Counteracts the Ability of Paclitaxel to Inhibit Cell Migration». Oncotarget. 2: 368–377.
- ^ Burgess J, Northcote DH (September 1969). «Action of colchicine and heavy water on the polymerization of microtubules in wheat root meristem». Journal of Cell Science. 5 (2): 433–51. doi:10.1242/jcs.5.2.433. PMID 5362335.
- ^ Mandelkow E, Mandelkow EM (February 1995). «Microtubules and microtubule-associated proteins». Current Opinion in Cell Biology. 7 (1): 72–81. doi:10.1016/0955-0674(95)80047-6. PMID 7755992.
- ^ Bramblett GT, Goedert M, Jakes R, Merrick SE, Trojanowski JQ, Lee VM (June 1993). «Abnormal tau phosphorylation at Ser396 in Alzheimer’s disease recapitulates development and contributes to reduced microtubule binding». Neuron. 10 (6): 1089–99. doi:10.1016/0896-6273(93)90057-X. PMID 8318230. S2CID 23180847.
- ^ «The Human Protein Atlas». www.proteinatlas.org. Archived from the original on 2017-05-01. Retrieved 2017-04-27.
- ^ Hirokawa, N (1994). «The neuronal cytoskeleton: roles in neuronal morphogenesis and organelle transport». Molecular Neurobiology: mechanisms common to brain, skin and immune system. Series: Progress in Clinical and Biological Research. Willey-Liss, Inc. 390: 117–143.
{{cite journal}}: CS1 maint: uses authors parameter (link) - ^ Hirokawa N, Noda Y, Tanaka Y, Niwa S (October 2009). «Kinesin superfamily motor proteins and intracellular transport». Nature Reviews. Molecular Cell Biology. 10 (10): 682–96. doi:10.1038/nrm2774. PMID 19773780. S2CID 18129292.
- ^ Marshall WF, Rosenbaum JL (March 1999). «Cell division: The renaissance of the centriole». Current Biology. 9 (6): R218–20. doi:10.1016/s0960-9822(99)80133-x. PMID 10209087. S2CID 16951268.
- ^ Pereira G, Schiebel E (February 1997). «Centrosome-microtubule nucleation». Journal of Cell Science. 110 (Pt 3): 295–300. doi:10.1242/jcs.110.3.295. PMID 9057082.
- ^ Hinchcliffe EH, Sluder G (May 2001). ««It takes two to tango»: understanding how centrosome duplication is regulated throughout the cell cycle». Genes & Development. 15 (10): 1167–81. doi:10.1101/gad.894001. PMID 11358861.
- ^ Forth S, Kapoor TM (June 2017). «The mechanics of microtubule networks in cell division». The Journal of Cell Biology. 216 (6): 1525–1531. doi:10.1083/jcb.201612064. PMC 5461028. PMID 28490474.
- ^ Khodjakov, A., Cole, R. W., Oakley, B. R. and Rieder, C. L. (2000). «Centrosome-independent mitotic spindle formation in vertebrates». Curr. Biol. 10, 59–67. doi:10.1016/S0960-9822(99)00276-6.
- ^ Rosenblatt J (March 2005). «Spindle assembly: asters part their separate ways». Nature Cell Biology. 7 (3): 219–22. doi:10.1038/ncb0305-219. PMID 15738974. S2CID 8082479.
- ^ Knoblich JA (December 2010). «Asymmetric cell division: recent developments and their implications for tumour biology». Nature Reviews. Molecular Cell Biology. 11 (12): 849–60. doi:10.1038/nrm3010. PMC 3941022. PMID 21102610.
- ^ Zhai Y, Kronebusch PJ, Borisy GG (November 1995). «Kinetochore microtubule dynamics and the metaphase-anaphase transition». The Journal of Cell Biology. 131 (3): 721–34. doi:10.1083/jcb.131.3.721. PMC 2120628. PMID 7593192.
- ^ Cai S, O’Connell CB, Khodjakov A, Walczak CE (July 2009). «Chromosome congression in the absence of kinetochore fibres». Nature Cell Biology. 11 (7): 832–8. doi:10.1038/ncb1890. PMC 2895821. PMID 19525938.
- ^ Bakhoum SF, Thompson SL, Manning AL, Compton DA (January 2009). «Genome stability is ensured by temporal control of kinetochore-microtubule dynamics». Nature Cell Biology. 11 (1): 27–35. doi:10.1038/ncb1809. PMC 2614462. PMID 19060894.
- ^ a b Meunier S, Vernos I (June 2012). «Microtubule assembly during mitosis — from distinct origins to distinct functions?». Journal of Cell Science. 125 (Pt 12): 2805–14. doi:10.1242/jcs.092429. PMID 22736044.
- ^ Mikhailov A, Gundersen GG (1998). «Relationship between microtubule dynamics and lamellipodium formation revealed by direct imaging of microtubules in cells treated with nocodazole or taxol». Cell Motility and the Cytoskeleton. 41 (4): 325–40. doi:10.1002/(SICI)1097-0169(1998)41:4<325::AID-CM5>3.0.CO;2-D. PMID 9858157.
- ^ Ren XD, Kiosses WB, Schwartz MA (February 1999). «Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton». The EMBO Journal. 18 (3): 578–85. doi:10.1093/emboj/18.3.578. PMC 1171150. PMID 9927417.
- ^ Waterman-Storer CM, Worthylake RA, Liu BP, Burridge K, Salmon ED (May 1999). «Microtubule growth activates Rac1 to promote lamellipodial protrusion in fibroblasts». Nature Cell Biology. 1 (1): 45–50. doi:10.1038/9018. PMID 10559863. S2CID 26321103.
- ^ Ezratty EJ, Partridge MA, Gundersen GG (June 2005). «Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase». Nature Cell Biology. 7 (6): 581–90. doi:10.1038/ncb1262. PMID 15895076. S2CID 37153935.
- ^ Ganguly A, Yang H, Sharma R, Patel KD, Cabral F (December 2012). «The role of microtubules and their dynamics in cell migration». The Journal of Biological Chemistry. 287 (52): 43359–69. doi:10.1074/jbc.M112.423905. PMC 3527923. PMID 23135278.
- ^ van Eeden F, St Johnston D (August 1999). «The polarisation of the anterior-posterior and dorsal-ventral axes during Drosophila oogenesis». Current Opinion in Genetics & Development. 9 (4): 396–404. doi:10.1016/S0959-437X(99)80060-4. PMID 10449356.
- ^ Beddington RS, Robertson EJ (January 1999). «Axis development and early asymmetry in mammals». Cell. 96 (2): 195–209. doi:10.1016/S0092-8674(00)80560-7. PMID 9988215. S2CID 16264083.
- ^ Tucker RP (1990). «The roles of microtubule-associated proteins in brain morphogenesis: a review». Brain Research. Brain Research Reviews. 15 (2): 101–20. doi:10.1016/0165-0173(90)90013-E. PMID 2282447. S2CID 12641708.
- ^ Rosette C, Karin M (March 1995). «Cytoskeletal control of gene expression: depolymerization of microtubules activates NF-kappa B». The Journal of Cell Biology. 128 (6): 1111–9. doi:10.1083/jcb.128.6.1111. PMC 2120413. PMID 7896875.
- ^ Ott C, Iwanciw D, Graness A, Giehl K, Goppelt-Struebe M (November 2003). «Modulation of the expression of connective tissue growth factor by alterations of the cytoskeleton». The Journal of Biological Chemistry. 278 (45): 44305–11. doi:10.1074/jbc.M309140200. PMID 12951326.
External links[edit]
- MBInfo — Microtubules
- 3D microtubule structures in the EM Data Bank(EMDB)
- Protocols for generating microtubules
Microtubules (MTs) are a protein–polymer that are a fundamental part of the cell cytoskeleton and are involved in many critical cellular processes, such as cell division, maintenance of cell polarity, and cargo transport (Amos & Schlieper, 2005; Desai & Mitchison, 1997; Howard & Hyman, 2003; Lansbergen & Akhmanova, 2006).
From: Methods in Cell Biology, 2013
Role of the cytoskeleton and membrane trafficking in axon–dendrite morphogenesis
Kevin C. Flynn, Frank Bradke, in Cellular Migration and Formation of Axons and Dendrites (Second Edition), 2020
2.3.3 Microtubules
Microtubules play important roles in many cellular functions, including neuronal morphogenesis. During neuronal development, microtubules must form stable bundles, which grow and reorganize to provide the main structural framework for the shafts of axons and dendrites. In fact, microtubules are the driving force underlying neurite extension. As with actin, many of the signaling pathways involved in neuronal polarization impinge upon proteins that modulate microtubule stability and dynamics. Furthermore, microtubules serve as the tracks for intracellular trafficking. Recent work has indicated that microtubules are actively regulated during neuronal polarization, changing in their dynamics, stability, and organization during axon formation and the subsequent neuronal morphogenesis (Hoogenraad and Bradke, 2009).
Microtubules are assembled from soluble tubulin dimers, which, like actin, can self-assemble into polymers (Desai and Mitchison, 1997; Box 2.2). Soluble tubulin exists as a heterodimer, consisting of α- and β-tubulin, which are the separate products from different genes and share about 50% amino acid homology. Furthermore, there are multiple isoforms of α- and β-tubulin, which can be differentially modulated by posttranslational modifications such as tyrosination, detyrosination, acetylation, polyglutamylation, and phosphorylation (Janke and Kneussel, 2010). The tubulin isoform composition of microtubules and the modifications they are subject to can influence the binding of microtubule-binding proteins, microtubule motors, and the dynamic properties of microtubules. An additional tubulin isoform, γ-tubulin shares around 30% homology with α- and β-tubulin (Moritz and Agard, 2001). γ-Tubulin is organized in large complexes that form an open ring structure, called the γ-tubulin ring complex (γTuRC), which plays an important role in microtubule nucleation (Raynaud-Messina and Merdes, 2007).
Box 2.2
Microtubule dynamics
During polymerization, the α/β-tubulin heterodimers arrange into linear protofilaments that associate laterally to form the hollow microtubule cylinders. In most mammalian cells, microtubules form a tube of 13 protofilaments. Within a protofilament, the tubulin heterodimers associate in a head-to-tail fashion. This makes microtubules intrinsically polar, resulting in two structurally and kinetically different ends: the highly dynamic plus end and the less dynamic minus end. The α-tubulin within the dimer is oriented toward the plus end, and the β-tubulin subunit toward the minus end (Desai and Mitchison, 1997; Howard and Hyman, 2003). Microtubules are intrinsically dynamic, a feature termed dynamic instability. They undergo periods of growth and shrinkage at the microtubule plus end. Dynamic instability allows microtubules to switch abruptly from growth to shrinkage (catastrophe) and from shrinkage to growth (rescue) (Mitchison and Kirschner, 1984; Howard and Hyman, 2003). The assembly and disassembly of microtubules are important for not only their generation but also their dynamic properties. The assembly of microtubules can be characterized by three steps: The first phase is defined by a thermodynamically unfavorable and therefore rate-limiting nucleation step. It is followed by rapid elongation of the polymer and finally by a steady-state phase. In the nucleation step, small oligomers of α/β-tubulin heterodimers form a nucleus. Once a stable oligomer of a certain size is reached, rapid polymerization of the microtubule occurs. During the steady state, microtubules display the dynamic instability, when microtubules switch randomly at their plus ends between “catastrophe” and “rescue,” leading to their highly dynamic behavior (Mitchison and Kirschner, 1984; Howard and Hyman, 2003). To maintain the dynamic instability, microtubules consume energy by the hydrolysis of GTP. β-Tubulin has a GTP-hydrolyzing activity that is strongly activated when the dimer is incorporated into the polymer. This hydrolyzing activity leads only to a small layer of tubulin dimers at the plus end that are bound to GTP, the so-called GTP cap. It stabilizes the plus end, because GDP-bound microtubules are intrinsically more unstable. If new polymerization is slower than the GTP hydrolysis, the plus end becomes unstable and results in catastrophe (Mitchison and Kirschner, 1984; Howard and Hyman, 2003). The dynamic behavior of the minus ends is not of interest in vivo, because they are generally capped and thus stabilized (Dammermann et al., 2003). Because GTP hydrolysis is not necessary for microtubule polymerization, the GTP hydrolysis is only important for the dynamic properties of microtubules.
Microtubules nucleate and polymerize spontaneously in vitro when α/β-tubulin concentrations are high. However, in cells, the intracellular monomer concentration seems too low for spontaneous nucleation, although this possibility has not been excluded (Job et al., 2003). Therefore, microtubule formation is assisted by specific structures called microtubule-organizing centers (MTOCs) (Luders and Stearns, 2007). MTOCs allow the cell to control where and when to assemble microtubules. The conventional MTOC in animal cells is the centrosome, an organelle next to the nucleus (see Chapter 2.5.5). Recently, also centrosome-independent and decentralized microtubule formation has been identified in many organisms and cell types (Bartolini and Gundersen, 2006; Luders and Stearns, 2007).
After assembly, individual microtubules assume a polarized tubule structure, which are arranged together into linear arrays in the axon. They are dynamic, yet rigid cylindrical, polymers of α/β-tubulin heterodimers with a diameter of about 25 nm (Box 2.2). Microtubules have a unique organization in neurons. In contrast to many somatic cells, neuronal microtubules are not anchored at the centrosome but are abundant in the cytoplasm throughout the whole cell body and funnel into the processes (Baas, 1999). The microtubules reach lengths up to 100 μm within the neurite shafts and are organized in regularly spaced, parallel arrays. During polymerization, the α/β-tubulin heterodimers arrange into linear protofilaments that associate laterally to form the hollow microtubule cylinders. After nucleation, microtubule minus ends are capped in cells, so that most of the interesting dynamics relevant for neuronal polarity occur at the plus ends. Microtubule plus ends oscillate between periods of slow growth and rapid shortening events called “catastrophies,” which can be “rescued” and growth reinitiated. This polymerization and depolymerization behavior is called dynamic instability (Box 2.2).
The polymerization and depolymerization dynamics of microtubules are critical for their cellular functions, including their role in neuronal morphogenesis (Conde and Caceres, 2009). In neurons, the number of microtubules growing distally into the peripheral domain of the growth cone is increased during rapid axon growth (Grabham et al., 2007, Fig. 2.3). In the growth cone, microtubules can grow methodically or rapidly disassemble and reorient their direction of growth. These features facilitate growth cone turning and axon guidance. Furthermore, microtubules can generate pushing forces during growth phases and pulling forces during shrinking phases that influence neurite elongation and retraction, respectively. Microtubule assembly and disassembly are regulated by various microtubule-binding proteins that can promote assembly, stabilize microtubules, or destabilize microtubules. Many of these proteins play an active role in neuronal polarization.
In addition to the modulation of microtubule dynamic instability, the structural regulation of microtubules into bundles is also essential for neuronal morphogenesis. Dense bundles of microtubules make up the main structural framework of both dendrites and axons. However, in mature neurons, axons and dendrites differ in their microtubule organization. In proximal dendrites, microtubules have a mixed polarity, with a population of plus ends facing the cell body and a population facing the distal dendrite. In axons, the microtubules predominantly have a plus-ends distal orientation. This difference is not the case before axonogenesis, where all neurites have uniform polarity microtubules with the plus ends distal. The conversion of the uniform polarity to mixed polarity microtubules in dendrites is a key process underlying axon–dendrite differentiation. It partially underlies differences in the growth behavior and the morphological differences of axons and dendrites. Furthermore, these differences in microtubule organization contribute to selective intracellular trafficking as discussed in the following.
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B978012814407700002X
ON THE RELATION BETWEEN MICROTUBULE DENSITY AND AXOPLASMIC TRANSPORT IN NERVES TREATED WITH MAYTANSINE IN VITRO
BERNARDINO GHETTI, SIDNEY OCHS, in Peripheral Neuropathies, 1978
Microtubules in unmyelinated fibers
The microtubule changes observed in unmyelinated fibers of nerves exposed to concentrations of MYT between 25 and 72 μM were more dramatic; all the axons in this concentration range were severely depleted of microtubules. In nerves exposed to concentrations of MYT between 4 and 25 μM, the microtubules, although present, were markedly reduced in number in comparison with control (Fig. 3A) unmyelinated axons. At concentrations below 4 μM the depletion of microtubules was still readily recognizable (Fig. 3B). It was much less severe in nerves exposed to concentrations of MYT below 1 μM, and at 0.05 μM MYT, the microtubule loss was not appreciable. An increase in the number of neurofilaments and the presence of an amorphous floccular material was observed in fibers exposed to MYT concentrations ranging from 3 to 72 μM. No paracrystalline structures were observed in the unmyelinated fibers at any of the MYT concentrations studied. The mean microtubule density of each of the control groups ranged from 61.5 to 86.5 microtubules/μm2. The mean microtubule density of the total control population of unmyelinated fibers was 78.13 microtubules/μm2. In fibers exposed to concentrations of MYT in the range from 17 to 72 εM, the mean density was 6.3% of the controls. In fibers exposed to concentrations of 5 μM the mean microtubule density was 8.85% of the controls. At 1 μM and 0.1 μM concentrations of MYT the microtubule densities were respectively 35.3% and 73.8% of control unmyelinated axons.
Fig. 3. A) Unmyelinated axons from a control nerve. Microtubules are clearly seen. B) Unmyelinated axons from a nerve exposed to 1 μM MYT. Note that the microtubules are markedly reduced in number.
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780444800794500211
Regulation of Nerve Fiber Elongation during Embryogenesis
PAUL C. LETOURNEAU, in Developmental Neuropsychobiology, 1986
2 Growth of the Cytoskeleton
Microtubules and neurofilaments are present all along the neurite, and many terminate in the growth cone. Microtubules always extend farther forward than neurofilaments and occasionally reach the bases of filopodia (Letourneau, 1979, 1983). This is significant because these cytoskeletal fibers grow by addition of subunits to an end and not by intercalation within a fiber (Kirschner, 1980; Margolis and Wilson, 1978). Thus, the ends of these fibers, particularly microtubules, may determine where the cytoskeleton is assembled (Heidemann et al., 1981). Because far more fibers terminate in the growth cone than elsewhere in the neurite, it maybe the major site for cytoskeletal growth in the elongating neurite, provided that sufficient tubulin monomer is present to drive polymerization at the distal microtubule ends (Letourneau, 1982).
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780123002716500081
Neurodevelopmental, neurocognitive, and behavioral disorders
Moyra Smith, in Mechanisms and Genetics of Neurodevelopmental Cognitive Disorders, 2021
4 Specific brain defects due to abnormalities in products of ciliary pathway genes
The structural and functional aspects of cilia and microtubules were described in Chapter 1.
In a 2017 review, Reiter and Leroux reported that 35 different ciliopathy disorders were known due to defects in 187 ciliopathy genes. In addition, 241 ciliopathy genes were potential candidate genes for ciliopathy disorders. They noted that both motile and immotile cilia were involved in ciliopathies. They distinguished first-order and second-order ciliopathies.
First-order ciliopathies included diseases resulting from defects in proteins in the ciliary basal body, or ciliary compartments and disorders in which there was disruption of intraflagellar transport.
Second-order ciliopathies were defined as disorders in which processes involved in cilia formation were disrupted.
It is important to note that in ciliopathies, a number of different tissues may be impacted. In this section, the focus will be on ciliopathies that primarily impact the nervous system. Reiter and Le Roux noted that an extensive array of organ systems can be impaired in ciliopathies. With respect to the brain, they reported that ciliopathies due to defects in nonmotile cilia can result in brain malformation, mental disabilities, epilepsy, and/or ataxia. Defects in the function of motile cilia can lead to hydrocephalus.
They also extensively reviewed the different cilia components and associated pathological defects that resulted from disruptions in genes that encode products in each ciliary component. Joubert syndrome can arise due to defects in products of any one of 15 different genes. In some cases, it results from digenic mutations.
Brain abnormalities that occur in Joubert syndrome include hypoplasia of the cerebellar vermis. A characteristic neuroimaging finding in Joubert syndrome is the so-called molar tooth abnormality. It is caused in part by hypoplasia or aplasia of the cerebellar vermis and reoriented cerebellar peduncles. The clinical neurological defects include ataxia, hypotonia, and oculomotor defects; changes in respiratory rhythm may be observed. Other systems that can be affected in Joubert syndrome include kidney and liver (Valente, 2013).
Stromme syndrome is a ciliopathy due to autosomal recessive mutations in a specific gene CENPF. In this syndrome, multiple systems can be affected, leading to cognitive defects, renal abnormalities, and intestinal atresia. CENPF protein is associated with mitotic spindles.
Several multisystem ciliopathies can also include brain defects. Bardet–Biedl syndrome can result from defects in any one of 19 different genes. This syndrome is reported to be autosomal recessively inherited. It is characterized by behavioral dysfunction and other features that may include obesity, kidney dysfunction, hypogonadism, polydactyly, retinitis pigmentosa, and intellectual impairment (Beales, 1999).
Centrosome and microtubules
Microtubules are involved in transport within cells. Microtubules are composed of different proteins including microtubule-associated proteins (MAPs) and alpha and beta tubulins. The centrosome forms the microtubule-organizing center. Microtubules play critical roles in cells division; they attach to the centromeres of chromosomes as the cell prepares for cell division. Microtubule abnormalities impact cell division and therefore represent a significant cause of microcephaly.
Microtubule centrosome and actin interactions
There is evidence that interactions between actin and microtubules in the cytoskeleton play important roles in cell polarity and in migration (Dogterom and Koenderink, 2019).
Dobyns (2018) noted that two proteins play roles as actin microtubule linkers. The proteins are microtubule and actin cross-linking factor 1 (MACF1) and dystonin (DST). They noted further that many different isoforms of MACF1 occur and that some isoforms are brain specific.
Dobyns et al. described a brain malformation that included lissencephaly, brain stem malformation, defective intercerebral connection, and hippocampal dysplasia. Individuals with these defects manifested intellectual disabilities, seizures, and aberrant muscle tone. Eight individuals with this disorder were found to have deleterious mutation in the MACF1 protein.
Microcephaly can arise due to defects in the following genes that encode microtubule-related products.
- •
-
MCPH1, microcephalin may play a role in G2/M checkpoint arrest in the cell cycle
- •
-
ASPM abnormal spindle microtubule assembly involved in mitotic spindle function
- •
-
CEP152, centrosomal protein 152 involved in centrosome function
- •
-
CEP63, centrosomal protein 63 involved in centrosome function
- •
-
NIN, ninein centrosomal protein 125 involved in centrosome function
- •
-
NDE1, nudE neurodevelopment protein 1
- •
-
CENPE, centromere protein E
- •
-
KIF5C, kinesin family member 5A functions as a microtubule motor
- •
-
KIF11, kinesin family member 11 involved in spindle dynamics
- •
-
TUBB2B, tubulin beta 2B class IIb binds GTP and is a major component of microtubules
- •
-
TUBB2A, tubulin beta 2A class IIa
- •
-
TUBG1, tubulin gamma 1 localizes to the centrosome forming ring complex
- •
-
POC1A, POC1 centriolar protein A plays important roles in basal body and cilia formation
- •
-
CENPJ, centromere protein J plays a structural role in the maintenance of centrosome integrity
Microcephaly centrosome and mitotic spindle defects
Alcantara and O’Driscoll (2014) documented clinical forms of microcephaly and specific centrosome or mitotic spindle defects that led to these disorders.
Clinical form of microcephaly, gene product mutated, structure impacted
- •
-
MCPH1, microcephalin, centrosome
- •
-
MCPH2 WDR62, mitotic spindle MCPH3 CDK5RAP2 centrosome
- •
-
MCPH3 CEP215, microtubule organization
- •
-
MCPH4 CASC5, spindle assembly kinetochore
- •
-
MCPH5 ASPM, spindle orientation
- •
-
MCPH6 CENJ, centriole biogenesis
- •
-
MCPH7 SCL/TAL1, centrosome centriole biogenesis
- •
-
MCPH8 CEP135, centrosome centriole biogenesis
- •
-
MCPH9 CEP152, centrosome biogenesis genome stability
It is important to note that microcephaly sometimes occurs in combination with defects in other body systems, and sometimes, microcephaly may occur in combination with growth retardation. Mutations in CEP63 (centrosomal protein 63) are reported to lead to Seckel syndrome with reduced growth and microcephaly.
In addition, microtubule and centrosome protein defects may occur in combination with other cortical malformations. Defects in the tubulin gamma complex, TUBG1, lead to microcephaly with complex cortical malformations.
Goncalves (2018), Vandervore (2019) reported that defects in the protein rotatin (RTTN) can lead to polymicrogyria (PMG) and microcephaly. Rotatin was reported to be involved in centriolar organization and ciliogenesis.
Mutations in tubulins and microtubular proteins lead to disorders sometimes referred to as tubulinopathies. Goncalves (2018) reported that brain imaging studies in individuals with tubulinopathies had revealed a range of different structural malformations that could impact basal ganglia, corpus callosum, and cerebellum. In some cases, tubulinopathies were associated with lissencephaly (smooth cortex with reduced gyri and sulci).
Shohayeb et al. (2019) reported that mutations in the WDR62 protein that is required for cilia formation are associated with microcephaly. They reported that WDR62 proteins within the basal body recruit proteins that are required for cilia formation, including centromere-associated J (CENPJ).
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B978012821913300007X
TUBULIN IN THE NERVOUS SYSTEM
ILLANA GOZES, … I. GOZES, in Selected Topics from Neurochemistry, 1985
MICROTUBULE FUNCTION IN THE NERVOUS SYSTEM: AXONAL GROWTH AND AXOPLASMIC TRANSPORT
Microtubules are present in all eukaryotic cells and have been found to play a variety of structural and dynamic roles in cell shape, division, motility, transport and secretion (Olmsted and Borisy, 1973). In nervous tissue, neurite outgrowth and transport in axons and dendrites are thought to be dependent on microtubule integrity (Schubert, Kreutzberg and Lux, 1972; Shelanski and Feit, 1972; Fink, Byers and Middaugh, 1973; Jeffrey and Austin, 1973). In the postnatal developing rat brain, the administration of colcemid (a colchicine analogue of a less toxic nature) results in a sparsity of glial cells and reduced dendritic growth and branching (Petit and Isaacson, 1977). In vitro, neurite extension of neuroblastoma cells was shown to be sensitive to colchicine and vinblastine (Seeds et al., 1970) and axonal elongation of sensory ganglia cells is also inhibited by colcemid (Roisen, Murphy and Braden, 1972). Thus, inhibition of microtubule assembly by antimitotic drugs results in the inhibition of axonal elongation. Slower axoplasmic transport rates as compared to the mature, faster rates characterize developing axons (Fink et al., 1973; Jeffrey and Austin, 1973). It is of interest to note that the process of axonal maturation coincides with an increase in the ability of tubulin to undergo self assembly (Fellous et al., 1975; Fellous, Francon, Lenon and Nunez, 1976; Schmitt et al., 1977b). The increase in the ability of tubulin to undergo self assembly might be due to both a decrease in microtubule assembly inhibitory activity (Koehn and Olsen, 1980) and to an increase in the concentration of active tau proteins, during brain development (Francon et al., 1978). The association of these proteins with the tubulin molecules might be facilitated by the changes in tubulin microheterogeneity observed during brain maturation (Gozes and Littauer, 1978; Dahl and Weibel, 1979).
In the mature axon the microtubule network moves along at the slowest rate of axoplasmic transport, 0.2–1 mm/day in mammals (Lasek, 1980; Black and Lasek, 1980). Colchicine and vinblastine inhibit axoplasmic transport (Wooten, Kopin and Axelrod, 1975) and cause depletion of neurotransmitters at the nerve terminals (Cheney, Hanin, Massarelli, Trabucchi and Costa, 1973). Microtubules are probably differentially associated with the various transport systems of different rates throughout the axon. Thus, for fast anterograde axoplasmic transport, in the presence of calcium, intact microtubules may not be required (Brady, Crothers, Nosal and McClure, 1980), while the slow axoplasmic transport system consists partially of microtubules (Black and Lasek, 1980). Different microtubules may have functional specificity along the neurites. Structurally different tubulin polymers can be formed in the presence of MAP2 or tau proteins when guanosine 5- (α, β methylene) triphosphate is included (Sandoval and Weber, 1980). In the slowly transported microtubules only tau proteins are identified but no high molecular weight microtubule-associated proteins are found (Tytell, Brady and Lasek, 1980).
Besides their involvement in axoplasmic transport microtubules have been implicated in synaptic transmission. MAP2 may be the protein that forms cross bridges between microtubules and specific organelles such as synaptic vesicles (Sattilaro et al., 1980). Other evidence that microtubules may be involved in synaptic transmission is that vinblastine depresses the postsynaptic evoked potential in the optic tectum after injection into the eyes of pigeons (Felder, 1975). In addition, colchicine inhibits the release of norepinephrine and dopamine β-hydroxylase from guinea pig vas deferens (Wooten et al., 1975). Colchicine and vinblastine were also found to inhibit the uptake of 5-hydroxytryptamine and norepinephrine by rat brain synaptosomes, while the uptake by isolated synaptic vesicles was not inhibited (Nomura and Segawa, 1975). In C. elegans neurons, Chalfie, M. and Thomson, J. N. have shown that the 15 protofilaments microtubules are required for sensory transduction but are not needed for the outgrowth of neuronal processes and 11 protofilament microtubules are involved in nerve growth and other microtubular functions in the cell but not sensory transduction (Cold Spring Harbor Symposium, 1981). These observations together with the finding of tubulin in the synaptic membrane (Kornguth and Sunderland, 1975) raise the possibility of the existence of a specific synaptic tubulin. Our data suggest that the tightly associated membrane tubulin in the plasmalemma as well as in the membranes of synaptic vesicle is enriched for α-tubulin subunits (Gozes and Littauer, 1979; Zisapel et al., 1980). As reviewed above, the tubulin in the membrane may be different from the cytoplasmic tubulin in its ability to accept tyrosine addition. Moreover, the degree of tubulin tyrosylation may modulate tubulin function.
Microtubules are also involved in neuronal shaping; destruction of the microtubular network results in disintegration of the neuronal processes. There has also been a suggestion that neuroblastoma cells recapitulate their detailed morphologies after reversible microtubule disassembly (Solomon, 1980).
It is interesting to note that some enzymatic activities in cells of the nervous system may depend on microtubule integrity, one example is ornithine decarboxylase (Gibbs, Hsu, Terasaki and Brooker, 1980).
In summary, microtubules seem to be intimately involved in the growth, and maintenance of neurons. Indeed, brain tissue from mentally retarded human patients, when examined by electron microscopy, is found to be deficient in microtubules (Gonatas, Evangelista and Walsh, 1967), or to have distended processes filled with haphazardly oriented microtubules and filaments (Gonatas and Goldensohn, 1965). Examination of brain tissue from mentally deficient human patients by light microscopy has revealed a sparsity of cortical neurons and glia (Marin-Padilla, 1974) as well as a reduction of dendritic growth and branching (Huttenlocher, 1974). In the Lesch-Nyhan syndrome, an x-linked inherited disease characterized by uricaciduria, mental retardation, compulsive self-mutilation, chloreopathetosis and spasticity, examination of the ultrastructure of platelets from a child with the disease showed depolymerized microtubules. Thus, malfunctioning microtubules might explain the neurological signs of Lesch-Nyhan syndrome (Martin, 1978). Malfunctioning microtubules in other tissues than brain were recently reviewed (Editorial, Lancet, 1978). Thus, deficiency in microtubules can occur under pathological conditions. Whether this constitutes the reason or the cause for the pathogenesis is still to be determined.
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780080319940500323
Neuroinflammation and neuroprotection in schizophrenia and autism spectrum disorder
Ehud Mekori-Domachevsky, … Raz Gross, in Neuroprotection in Autism, Schizophrenia and Alzheimer’s Disease, 2020
Abstract
Microtubules are dynamic polymers essential in the proper development and maintenance of a healthy nervous system. Previously, increasing evidence linked the defective regulation of microtubules to a spectrum of disorders from neurodevelopmental to neurodegenerative diseases. Acetylation of tubulin determines the biochemical and biophysical diversity of microtubules, regulates their function, and has been recently related to the molecular events underlying different disorders including schizophrenia, autism, and Alzheimer’s disease. Here, we critically look at the experimental data coming from in vitro to in vivo disease models and patients with the aim of understanding whether targeting tubulin acetylation could be a promising strategy for neuroprotection. We conclude that this is a feasible road, but in the future, a more comprehensive analysis of molecular details at the base of tubulin acetylation and, most important, the consequent determination of novel compounds targeting this process are the condition sine qua non for moving toward therapeutic interventions.
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780128140376000112
Neuronal migration in the developing cerebellar system
Christophe Laumonnerie, David J. Solecki, in Cellular Migration and Formation of Axons and Dendrites (Second Edition), 2020
19.1.2.3 The roles of the microtubule cytoskeleton and associated motors
Microtubules are cylindrical polymers of the α- and β-tubulin proteins that form a cytoskeletal system providing structural support in the cell cytoplasm, as well as an architectural network by which molecular machines called motor proteins can perform physical tasks (e.g., segregation of chromosomes during mitosis or material and organelle transport) (Watanabe et al., 2005; Etienne-Manneville, 2013; Borisy et al., 2016). Although EM analyses noted microtubules in the CGN leading process in vivo and after correlated light and EM microscopy (Rakic, 1971; Gregory et al., 1988), the acquisition of an overall picture of the microtubule cytoskeleton in migrating CGNs had to await the development of improved microtubule labeling techniques. Tubulin antibody staining revealed that bundled microtubules are located in a central region of the leading process and in a “cage-like web of filaments” surrounding the CGN nucleus (Rivas and Hatten, 1995) (Fig. 19.5A) that sometimes appears as a forklike microtubule structure, depending on the method of fixation used and/or the neuronal cell type (Xie et al., 2003). Labeling microtubules with a genetically encoded fluorescent protein tag highlighted alterations to the microtubule cage during two-stroke motility: During forward movement, perinuclear microtubules undergo dynamic shape fluctuations, but they continually encircle the nucleus as the cage and nucleus move as a unit (Solecki et al., 2004). Just before cell body translocation, the cage stretches and a group of microtubules physically enter the proximal leading process (Fig. 19.5B). During translocation, the microtubules in the cage are compressed because of the forward nuclear movement, resulting in a compact profile that perdures until the next saltatory round of two-stroke motility. Umeshima et al. confirmed that the nuclei of CGNs migrating in ex vivo slice preparations are surrounded by a cage of microtubules (Umeshima et al., 2007). They further showed that the cage is composed of different populations of microtubules: (1) a tyrosinated population representing the significant cage fraction that is unstable and (2) an acetylated population around the anterior surface of the nucleus that is stable. Surprisingly, acetylated microtubules extended from the nucleus toward the proximal leading process and were not associated with the centrosomes as previously thought. The dissolution of these microtubule-based structures by pharmacological manipulation with drugs such as nocodazole or colchicine blocks the movement of neurons throughout the brain by destabilizing their leading and trailing processes and ultimately perturbs two-stroke motility, illustrating the importance of the microtubule-based cytoskeleton for neuronal migration.
Figure 19.5. The CGN microtubule cytoskeleton and the roles of microtubule motors.
(A) Tubulin antibody staining reveals a cage-like lattice of microtubules that surrounds the CGN nucleus. Inset: shows a higher magnification view of the CGN cage. (B) Dynamics of the CGN microtubule cytoskeleton. A Venus-Tubulin construct was introduced into CGNs and high-speed time-lapse imaging used to examine morphological changes. In Phase I the cage is compact, in Phase II it stretches, and in Phase III the cage is deformed as the nucleus advances in the cell body. lp, leading process. (C) Diagram of the role of microtubule motors in CGN migration. Increased expression of KIF11 prevents microtubule slide to lock CGNs in a stationary phase of migration. In Phase II of the CGN migration cycle, cytoplasmic dynein motors in the leading process help guide the centrosome into the leading process. In Phase III of the cycle, nuclear associated cytoplasmic dynein motors transport the nucleus forward to the centrosome while kinesin motors provide additional force to rotate the nucleus during forward movement.
(A) and (B) adapted with permission from Solecki D.J., Model, L., Gaetz, J., Kapoor, T.M., Hatten, M.E., Par6alpha signaling controls glial-guided neuronal migration. Nat. Neurosci. 2004;7(11):1195–1203).
Insights gained from the positional cloning of genes in which mutations cause the human neuronal migration disorder called lissencephaly and from their subsequent genetic manipulation in mice have provided a compelling entry point through which to understand the roles of microtubules and some microtubule motor proteins in neuronal migration during brain lamination (Gleeson and Walsh, 2000; Ross and Walsh, 2001; Kato and Dobyns, 2003; Metin et al., 2008). Isolated lissencephaly syndrome and Miller–Dieker syndrome are rare brain disorders characterized by a smooth-appearing brain with defective lamination, intractable epilepsy, and apparent defects in neuronal migration that are associated with spontaneous deletions in the vicinity of the PAFAH1B1 gene on the short arm of chromosome 17 (Dobyns et al., 1993; Reiner et al., 1993). PAFAH1B1 encodes the Lis1 protein, which is an evolutionarily conserved, essential cofactor for the cytoplasmic dynein minus end–directed microtubule motor protein (Morris et al., 1998; Efimov and Morris, 2000; Faulkner et al., 2000; Smith et al., 2000; Morris, 2003; Shu et al., 2004; Tsai et al., 2005; Tsai et al., 2007). The genetic deletion of PAFAH1B1 (Hirotsune et al., 1998) or a series of genetically and biochemically interacting proteins (Umeshima et al., 2007) leads not only to profound inhibition of all microtubule minus end–directed dynein transport but also to CGN migration defects, including stalled motility of CGNs in microculture and in their movement to the IGL in vivo. Interestingly, the loss of Lis1 function perturbed two-stroke nucleokinesis in both CGNs and cortical pyramidal neurons in a unique fashion: in both cell types, nuclear displacement was considerably slowed and the progressive centrosome/cell body advance was affected, although centrosome movement was randomized, as opposed to being halted completely (Tsai et al., 2007; Umeshima et al., 2007). These results led to the formulation of a model of a dual role for dynein activity, whereby dynein is tethered at the cell cortex in the leading process and, as in the yeast model system (Dujardin and Vallee, 2002), helps guide the centrosome forward in the first stage of the two-stroke cycle, whereas dynein on the nuclear envelope serves to move the nucleus toward the centrosome in the final stage of the two-stroke nucleokinesis model (Tsai et al., 2007; Vallee et al., 2009) (Fig. 19.5C).
In contrast to the single dynein motor that moves cargos in a minus end–directed manner along cytoplasmic microtubules, scores of kinesins move cargos in the opposite direction to the plus end (Vallee et al., 2004; Hook and Vallee, 2006; Hirokawa et al., 2009; Vallee et al., 2009; Hirokawa et al., 2010; Bachmann and Straube, 2015). Given the assortment of kinesins that have been identified, it is not surprising that various kinesin-related functions concern the two-stroke nucleokinesis system (Fig. 19.5C). For example, KIF11 is expressed in CGNs that have reached their final destination in the IGL in various cell biological models, including the mitotic spindle and extending axons. KIF11 is a slow kinesin motor that acts as an antagonist of dynein-dependent transport (Falnikar et al., 2011). Pharmacological inhibition of KIF11 and gene silencing stimulate CGN migration both in vitro and in ex vivo slices, potentially by stimulating microtubule sliding. This suggests that high KIF11 activity stalls migration once CGNs have reached the IGL. As a second example, KIF6 loss of function strongly diminished the directional persistence and boosted the randomized migration directions of CGNs while simultaneously stimulating the appearance of multiple leading process–like extensions, implicating the defective polarized leading-process morphology in random motility (Falnikar et al., 2013). Remarkably, KIF6 cooperates with the MgcRacGAP protein in CGNs as it does in the centralspindlin complex of cells undergoing cytokinesis, suggesting that the well-characterized cell biological mechanisms of the centralspindlin complex in cytokinesis are recycled for leading-process morphogenesis in migrating neurons. In a final example, kinesin motors cooperate with dynein to regulate the efficiency of the nucleokinesis phase of the two-stroke motility. Interestingly, Wu and colleagues discovered that the nuclei of migrating CGNs tend to rotate on an axis parallel to their migration direction before or during the last phase of the movement cycle, whereas stationary or postmigratory nuclei display no such rotation (Wu et al., 2018). Moreover, nuclear rotations were accompanied by the formations of “sharp peaks” at the leading edge of the nucleus, indicating that localized pulling forces were applied to the moving nuclei. The individual inhibition of dynein and KIF5B not only strongly affected nuclear rotation but also decreased the overall displacement of migrating CGNs and abrogated the apparent pulling forces applied to the leading edge of the nuclei, suggesting that coordination between plus- and minus-end motors is required for the optimal application of force to the large neuronal nucleus during CGN motility. Taken together, the results of these studies implicate the motor proteins that drive microtubule-dependent organelle transport as playing key roles in driving the two-stroke motility of migrating CGNs.
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780128144077000195
Dendrite development: invertebrates
Wesley B. Grueber, Bing Ye, in Cellular Migration and Formation of Axons and Dendrites (Second Edition), 2020
11.4.2 Dynein-dependent trafficking controls dendritic branching
Microtubule polarity influences the trafficking of proteins and organelles throughout the cell. Microtubule cargos are trafficked by microtubule motors, and different types of motors move with either minus-end or plus-end directed polarity. The kinesin family of proteins includes the major plus-end motors, whereas dyneins carry cargos in a minus-end direction. Dynein, a very large protein complex, is the major microtubule motor for dendrites. The dynein complex consists of core components: dynein heavy chain, dynein light chain, light intermediate chain (Dlic), and light chain. Both dynein and dynein cargos are important for the spatial distribution of branches along a dendritic arbor (Zheng et al., 2008; Satoh et al., 2008). Dlic was identified in mutagenesis screens for genes affecting dendritic morphogenesis. These studies of Dlic focused on a group of neurons, the da neurons that have branches concentrated near the margins of the arbor but are sparse in the center of the receptive field near the cell body (Fig. 11.2A). Neurons lacking Dlic show an aberrant shift of dendritic branches from distal regions of the arbor to proximal regions, suggesting that trafficking of branching machinery is normally required to place branches in specific locations along an arbor and is disrupted in neurons lacking Dlic (Fig. 11.2B). The dynein complex is essential for the distribution of dendritic Golgi outposts, which regulate dendritic branching (Ye et al., 2007; Zheng et al., 2008) (see details below). In addition, disruption of the Rab5 protein, a small GTPase of the Rab family and a regulator of the early endocytic pathway, in Dlic mutant neurons blocks the proximal hyperbranching Dlic phenotype without restoring branching at the margins of the arbor (Fig. 11.2C). Thus, Golgi outposts and Rab5 are components of the trafficking machinery required for dendritic arbor elaboration (Zheng et al., 2008; Satoh et al., 2008).
Figure 11.2. Spatial control of branching by dynein trafficking. (A) Wild-type class IV arbor shows branching most concentrated near the margin of the arbor (arrow) and more sparse branching nearer to the cell body (arrowhead). (B) In mutants of Dynein light intermediate chain 2 (Dlic2), the arborization becomes concentrated near the cell body, with distal dendrites showing poor elaboration. (C) Expression of a dominant negative (DN) Rab5 construct in Dlic2 neurons suppresses the proximal branching phenotype.
Data are summarized from Satoh, D., Sato, D., Tsuyama, T., Saito, M., Ohkura, H., Rolls, M. M., Ishikawa, F., Uemura, T., 2008. Spatial control of branching within dendritic arbors by dynein-dependent transport of Rab5-endosomes. Nat. Cell Biol. 10, 1164–1171; Zheng, Y., Wildonger, J., Ye, B., Zhang, Y., Kita, A., Younger, S.H., Zimmerman, S., Jan, L.Y., Jan, Y.N., 2008. Dynein is required for polarized dendritic transport and uniform microtubule orientation in axons. Nat. Cell Biol. 10, 1172–1180.
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780128144077000110
Radial migration in the developing cerebral cortex
Stephen C. Noctor, … Arnold R. Kriegstein, in Cellular Migration and Formation of Axons and Dendrites (Second Edition), 2020
15.7.4.1 Lis1
Microtubule organization in migrating neurons is regulated by several factors including platelet-activating factor acetylhydrolase isoform 1b regulatory subunit 1 (PAFAH1B1, or LIS1) (Moon and Wynshaw-Boris, 2013). Mutations in the LIS1 gene cause lissencephaly in humans (Reiner et al., 1993), a brain malformation characterized by a smooth cerebral cortex, lack of sulci and gyri, and disrupted cortical layers. Mutant Lis1 KO mice present with delayed neuronal migration in heterozygote KO animals and a more dramatic phenotype in homozygote KOs (Hirotsune et al., 1998). Lis1 knockdown through in utero RNA interference (RNAi) induces an accumulation of multipolar neurons in the SVZ of the embryonic cortex (Tsai et al., 2005). Lis1 is a microtubule-associated protein that binds tubulin and other microtubule regulatory proteins (Sapir et al., 1997) including Dynein/Dynactin (Faulkner et al., 2000; Smith et al., 2000), Ndel1/NUDEL (Sasaki et al., 2000), and Nde1/mNudE (Feng et al., 2000).
Lis1 is thought to induce its effects by promoting dynein ATPase activity (Mesngon et al., 2006). Genetic knockout or RNAi knockdown of Ndel1, which is thought to facilitate the interaction of Lis1 and dynein, prevents many cells from migrating away from the proliferative zones (Sasaki et al., 2005). Lis1 also interacts with actin, as haploinsufficiency of Lis1 in granule neurons leads to reduced F-actin in ends of processes (Kholmanskikh et al., 2003). Evidence shows cross talk between Lis1 and Reelin signaling that regulates cellular migration. The Alpha 1 and 2 subunits of the Pafah1b complex bind to the VLDLR (Assadi et al., 2003; Zhang et al., 2007), which promotes its interaction with Lis1 (Chai and Frotscher, 2016), making this an important node in the regulation of neuronal migration (Moon and Wynshaw-Boris, 2013).
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780128144077000158
CALMODULIN-BINDING PROTEINS IN BRAIN
SHIRO KAKIUCHI, in Selected Topics from Neurochemistry, 1985
6 CALMODULIN-BINDING PROTEINS OF BRAIN MICROTUBULES
Although cytoplasmic microtubules are mainly composed of tubulin (about 85%), they also contain a number of other proteins (about 15%) as ‘microtubule-associated proteins’ (MAPs). These non-tubulin accessory proteins are present in microtubules in roughly constant stoichiometry with tubulin through multiple cycles of assembly-disassembly. A possibility that these MAPs are involved in the regulation of microtubule assembly in vivo has deserved attention recently because when the MAPs are separated from the tubulin by phosphocellulose column chromatography, the tubulin no longer assembles into microtubules and addition of the MAPs back to the tubulin fully restored the capacity of tubulin to assemble (for a review see Timasheff and Grisham, 1980). Two MAP species have been characterized recently. These are identified on SDS-polyacrylamide gel electrophoresis as two major high molecular weight (~ 300,000) bands (HMW-MAPs) and a family of four closely related low molecular weight (55,000–62,000) bands. The latter polypeptides (Mr = 55,000–62,000) were collectively termed tau (τ) factor by Weingarten, Lockwood, Hwo and Kirschner (1975).
There is now much evidence that the concentration of Ca2+ regulates the assembly-disassembly of microtubules in the cell: increase and decrease in the Ca2+ concentration causes disassembly and assembly, respectively (see Timasheff and Grisham, 1980). Therefore, an interesting possibility is the involvement of calmodulin in the regulation of microtubule assembly as a mediator of the action of Ca2+. Indeed, Marcum, Dedman, Brinkley and Means (1978) and Nishida, Kumagai, Ohtsuki and Sakai (1979) independently observed that calmodulin both inhibits and reverses microtubule assembly in the presence of Ca2+. Moreover, the immunofluorescence localization of calmodulin in the dividing cell revealed that it is associated with the chromosome-to-pole region of the mitotic apparatus, where the disassembly of microtubules takes place (Welsh, Dedman, Brinkley and Means, 1978, 1979). In subsequent studies using microtubules purified from brain, Kumagai and Nishida (1979, 1980) demonstrated the Ca2+-dependent complex formation between calmodulin and tubulin on Sephadex G-200 gel filtration column chromatography and high speed liquid column chromatography. The complex thus formed was incapable of polymerizing into microtubules. Quantitative analysis of the binding revealed that 2 mol of calmodulin can bind to 1 mol of tubulin with a dissociation constant of about 4 μM (Kumagai, Nishida and Sakai, 1982).
In an independent study, Sobue, Fujita, Muramoto and Kakiuchi (1981a) found that the tau (τ) factor, one species of the microtubule associated proteins (see above), is a calmodulin-binding protein undergoing a Ca2+-dependent association with calmodulin. Subsequently, they reconstituted with purified tubulin, τ factor and calmodulin a Ca2+-sensitive microtubule assembly system (Kakiuchi and Sobue, 1981). In this system, raising the Ca2+ concentration produced the inhibition of assembly by forming a calmodulin-τ factor complex. Thus, τ protein acted as a flip-flop switch forming complexes between calmodulin at the increased Ca2+ level and tubulin at the decreased Ca2+ level thereby leading to disassembly and assembly, respectively, of microtubules (Fig. 1). It is unclear at present whether calmodulin, in the presence of Ca2+, interacts in vivo with both tubulin and τ factor or either of the two proteins for the microtubule disassembly. This point should be clarified in the future study.
Fig. 1. Ca2+-dependent and τ factor-linked flip-flop regulation of microtubule assembly (Kakiuchi and Sobue, 1981). CaM, calmodulin; T, tubulin.
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780080319940500335