From Wikipedia, the free encyclopedia
This article is about chemical synapses of the nervous system. For general information, see synapse. For other uses, see synapse (disambiguation).
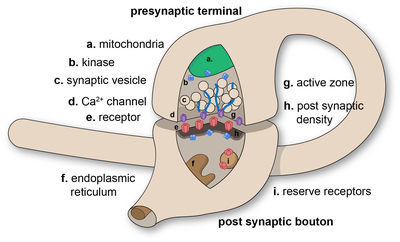
Artistic interpretation of the major elements in chemical synaptic transmission. An electrochemical wave called an action potential travels along the axon of a neuron. When the action potential reaches the presynaptic terminal, it provokes the release of a synaptic vesicle, secreting its quanta of neurotransmitter molecules. The neurotransmitter binds to chemical receptor molecules located in the membrane of another neuron, the postsynaptic neuron, on the opposite side of the synaptic cleft.
Chemical synapses are biological junctions through which neurons’ signals can be sent to each other and to non-neuronal cells such as those in muscles or glands. Chemical synapses allow neurons to form circuits within the central nervous system. They are crucial to the biological computations that underlie perception and thought. They allow the nervous system to connect to and control other systems of the body.
At a chemical synapse, one neuron releases neurotransmitter molecules into a small space (the synaptic cleft) that is adjacent to another neuron. The neurotransmitters are contained within small sacs called synaptic vesicles, and are released into the synaptic cleft by exocytosis. These molecules then bind to neurotransmitter receptors on the postsynaptic cell. Finally, the neurotransmitters are cleared from the synapse through one of several potential mechanisms including enzymatic degradation or re-uptake by specific transporters either on the presynaptic cell or on some other neuroglia to terminate the action of the neurotransmitter.
The adult human brain is estimated to contain from 1014 to 5 × 1014 (100–500 trillion) synapses.[1] Every cubic millimeter of cerebral cortex contains roughly a billion (short scale, i.e. 109) of them.[2] The number of synapses in the human cerebral cortex has separately been estimated at 0.15 quadrillion (150 trillion)[3]
The word «synapse» was introduced by Sir Charles Scott Sherrington in 1897.[4] Chemical synapses are not the only type of biological synapse: electrical and immunological synapses also exist. Without a qualifier, however, «synapse» commonly refers to chemical synapse.
Structure[edit]
Diagram of a chemical synaptic connection
Further information on formation of synapses: Synaptogenesis
Synapses are functional connections between neurons, or between neurons and other types of cells.[5][6] A typical neuron gives rise to several thousand synapses, although there are some types that make far fewer.[7] Most synapses connect axons to dendrites,[8][9] but there are also other types of connections, including axon-to-cell-body,[10][11] axon-to-axon,[10][11] and dendrite-to-dendrite.[9] Synapses are generally too small to be recognizable using a light microscope except as points where the membranes of two cells appear to touch, but their cellular elements can be visualized clearly using an electron microscope.
Chemical synapses pass information directionally from a presynaptic cell to a postsynaptic cell and are therefore asymmetric in structure and function. The presynaptic axon terminal, or synaptic bouton, is a specialized area within the axon of the presynaptic cell that contains neurotransmitters enclosed in small membrane-bound spheres called synaptic vesicles (as well as a number of other supporting structures and organelles, such as mitochondria and endoplasmic reticulum). Synaptic vesicles are docked at the presynaptic plasma membrane at regions called active zones.
Immediately opposite is a region of the postsynaptic cell containing neurotransmitter receptors; for synapses between two neurons the postsynaptic region may be found on the dendrites or cell body. Immediately behind the postsynaptic membrane is an elaborate complex of interlinked proteins called the postsynaptic density (PSD).
Proteins in the PSD are involved in anchoring and trafficking neurotransmitter receptors and modulating the activity of these receptors. The receptors and PSDs are often found in specialized protrusions from the main dendritic shaft called dendritic spines.
Synapses may be described as symmetric or asymmetric. When examined under an electron microscope, asymmetric synapses are characterized by rounded vesicles in the presynaptic cell, and a prominent postsynaptic density. Asymmetric synapses are typically excitatory. Symmetric synapses in contrast have flattened or elongated vesicles, and do not contain a prominent postsynaptic density. Symmetric synapses are typically inhibitory.
The synaptic cleft—also called synaptic gap—is a gap between the pre- and postsynaptic cells that is about 20 nm (0.02 μ) wide.[12] The small volume of the cleft allows neurotransmitter concentration to be raised and lowered rapidly.[13]
An autapse is a chemical (or electrical) synapse formed when the axon of one neuron synapses with its own dendrites.
Signaling in chemical synapses[edit]
Overview[edit]
Here is a summary of the sequence of events that take place in synaptic transmission from a presynaptic neuron to a postsynaptic cell. Each step is explained in more detail below. Note that with the exception of the final step, the entire process may run only a few hundred microseconds, in the fastest synapses.[14]
- The process begins with a wave of electrochemical excitation called an action potential traveling along the membrane of the presynaptic cell, until it reaches the synapse.
- The electrical depolarization of the membrane at the synapse causes channels to open that are permeable to calcium ions.
- Calcium ions flow through the presynaptic membrane, rapidly increasing the calcium concentration in the interior.
- The high calcium concentration activates a set of calcium-sensitive proteins attached to vesicles that contain a neurotransmitter chemical.
- These proteins change shape, causing the membranes of some «docked» vesicles to fuse with the membrane of the presynaptic cell, thereby opening the vesicles and dumping their neurotransmitter contents into the synaptic cleft, the narrow space between the membranes of the pre- and postsynaptic cells.
- The neurotransmitter diffuses within the cleft. Some of it escapes, but some of it binds to chemical receptor molecules located on the membrane of the postsynaptic cell.
- The binding of neurotransmitter causes the receptor molecule to be activated in some way. Several types of activation are possible, as described in more detail below. In any case, this is the key step by which the synaptic process affects the behavior of the postsynaptic cell.
- Due to thermal vibration, the motion of atoms, vibrating about their equilibrium positions in a crystalline solid, neurotransmitter molecules eventually break loose from the receptors and drift away.
- The neurotransmitter is either reabsorbed by the presynaptic cell, and then repackaged for future release, or else it is broken down metabolically.
Neurotransmitter release[edit]
Release of neurotransmitter occurs at the end of axonal branches.
The release of a neurotransmitter is triggered by the arrival of a nerve impulse (or action potential) and occurs through an unusually rapid process of cellular secretion (exocytosis). Within the presynaptic nerve terminal, vesicles containing neurotransmitter are localized near the synaptic membrane. The arriving action potential produces an influx of calcium ions through voltage-dependent, calcium-selective ion channels at the down stroke of the action potential (tail current).[15] Calcium ions then bind to synaptotagmin proteins found within the membranes of the synaptic vesicles, allowing the vesicles to fuse with the presynaptic membrane.[16] The fusion of a vesicle is a stochastic process, leading to frequent failure of synaptic transmission at the very small synapses that are typical for the central nervous system. Large chemical synapses (e.g. the neuromuscular junction), on the other hand, have a synaptic release probability, in effect, of 1. Vesicle fusion is driven by the action of a set of proteins in the presynaptic terminal known as SNAREs. As a whole, the protein complex or structure that mediates the docking and fusion of presynaptic vesicles is called the active zone.[17] The membrane added by the fusion process is later retrieved by endocytosis and recycled for the formation of fresh neurotransmitter-filled vesicles.
An exception to the general trend of neurotransmitter release by vesicular fusion is found in the type II receptor cells of mammalian taste buds. Here the neurotransmitter ATP is released directly from the cytoplasm into the synaptic cleft via voltage gated channels.[18]
Receptor binding[edit]
Receptors on the opposite side of the synaptic gap bind neurotransmitter molecules. Receptors can respond in either of two general ways. First, the receptors may directly open ligand-gated ion channels in the postsynaptic cell membrane, causing ions to enter or exit the cell and changing the local transmembrane potential.[14] The resulting change in voltage is called a postsynaptic potential. In general, the result is excitatory in the case of depolarizing currents, and inhibitory in the case of hyperpolarizing currents. Whether a synapse is excitatory or inhibitory depends on what type(s) of ion channel conduct the postsynaptic current(s), which in turn is a function of the type of receptors and neurotransmitter employed at the synapse. The second way a receptor can affect membrane potential is by modulating the production of chemical messengers inside the postsynaptic neuron. These second messengers can then amplify the inhibitory or excitatory response to neurotransmitters.[14]
Termination[edit]
After a neurotransmitter molecule binds to a receptor molecule, it must be removed to allow for the postsynaptic membrane to continue to relay subsequent EPSPs and/or IPSPs. This removal can happen through one or more processes:
- The neurotransmitter may diffuse away due to thermally-induced oscillations of both it and the receptor, making it available to be broken down metabolically outside the neuron or to be reabsorbed.[19]
- Enzymes within the subsynaptic membrane may inactivate/metabolize the neurotransmitter.
- Reuptake pumps may actively pump the neurotransmitter back into the presynaptic axon terminal for reprocessing and re-release following a later action potential.[19]
Synaptic strength[edit]
The strength of a synapse has been defined by Sir Bernard Katz as the product of (presynaptic) release probability pr, quantal size q (the postsynaptic response to the release of a single neurotransmitter vesicle, a ‘quantum’), and n, the number of release sites. «Unitary connection» usually refers to an unknown number of individual synapses connecting a presynaptic neuron to a postsynaptic neuron.
The amplitude of postsynaptic potentials (PSPs) can be as low as 0.4 mV to as high as 20 mV.[20] The amplitude of a PSP can be modulated by neuromodulators or can change as a result of previous activity. Changes in the synaptic strength can be short-term, lasting seconds to minutes, or long-term (long-term potentiation, or LTP), lasting hours. Learning and memory are believed to result from long-term changes in synaptic strength, via a mechanism known as synaptic plasticity.
Receptor desensitization[edit]
Desensitization of the postsynaptic receptors is a decrease in response to the same neurotransmitter stimulus. It means that the strength of a synapse may in effect diminish as a train of action potentials arrive in rapid succession – a phenomenon that gives rise to the so-called frequency dependence of synapses. The nervous system exploits this property for computational purposes, and can tune its synapses through such means as phosphorylation of the proteins involved.
Synaptic plasticity[edit]
Synaptic transmission can be changed by previous activity. These changes are called synaptic plasticity and may result in either a decrease in the efficacy of the synapse, called depression, or an increase in efficacy, called potentiation. These changes can either be long-term or short-term. Forms of short-term plasticity include synaptic fatigue or depression and synaptic augmentation. Forms of long-term plasticity include long-term depression and long-term potentiation. Synaptic plasticity can be either homosynaptic (occurring at a single synapse) or heterosynaptic (occurring at multiple synapses).
Homosynaptic plasticity[edit]
Homosynaptic plasticity (or also homotropic modulation) is a change in the synaptic strength that results from the history of activity at a particular synapse. This can result from changes in presynaptic calcium as well as feedback onto presynaptic receptors, i.e. a form of autocrine signaling. Homosynaptic plasticity can affect the number and replenishment rate of vesicles or it can affect the relationship between calcium and vesicle release. Homosynaptic plasticity can also be postsynaptic in nature. It can result in either an increase or decrease in synaptic strength.
One example is neurons of the sympathetic nervous system (SNS), which release noradrenaline, which, besides affecting postsynaptic receptors, also affects presynaptic α2-adrenergic receptors, inhibiting further release of noradrenaline.[21] This effect is utilized with clonidine to perform inhibitory effects on the SNS.
Heterosynaptic plasticity[edit]
Heterosynaptic plasticity (or also heterotropic modulation) is a change in synaptic strength that results from the activity of other neurons. Again, the plasticity can alter the number of vesicles or their replenishment rate or the relationship between calcium and vesicle release. Additionally, it could directly affect calcium influx. Heterosynaptic plasticity can also be postsynaptic in nature, affecting receptor sensitivity.
One example is again neurons of the sympathetic nervous system, which release noradrenaline, which, in addition, generates an inhibitory effect on presynaptic terminals of neurons of the parasympathetic nervous system.[21]
Integration of synaptic inputs[edit]
In general, if an excitatory synapse is strong enough, an action potential in the presynaptic neuron will trigger an action potential in the postsynaptic cell. In many cases the excitatory postsynaptic potential (EPSP) will not reach the threshold for eliciting an action potential. When action potentials from multiple presynaptic neurons fire simultaneously, or if a single presynaptic neuron fires at a high enough frequency, the EPSPs can overlap and summate. If enough EPSPs overlap, the summated EPSP can reach the threshold for initiating an action potential. This process is known as summation, and can serve as a high pass filter for neurons.[22]
On the other hand, a presynaptic neuron releasing an inhibitory neurotransmitter, such as GABA, can cause an inhibitory postsynaptic potential (IPSP) in the postsynaptic neuron, bringing the membrane potential farther away from the threshold, decreasing its excitability and making it more difficult for the neuron to initiate an action potential. If an IPSP overlaps with an EPSP, the IPSP can in many cases prevent the neuron from firing an action potential. In this way, the output of a neuron may depend on the input of many different neurons, each of which may have a different degree of influence, depending on the strength and type of synapse with that neuron. John Carew Eccles performed some of the important early experiments on synaptic integration, for which he received the Nobel Prize for Physiology or Medicine in 1963.
Volume transmission[edit]
When a neurotransmitter is released at a synapse, it reaches its highest concentration inside the narrow space of the synaptic cleft, but some of it is certain to diffuse away before being reabsorbed or broken down. If it diffuses away, it has the potential to activate receptors that are located either at other synapses or on the membrane away from any synapse. The extrasynaptic activity of a neurotransmitter is known as volume transmission.[23] It is well established that such effects occur to some degree, but their functional importance has long been a matter of controversy.[24]
Recent work indicates that volume transmission may be the predominant mode of interaction for some special types of neurons. In the mammalian cerebral cortex, a class of neurons called neurogliaform cells can inhibit other nearby cortical neurons by releasing the neurotransmitter GABA into the extracellular space.[25] Along the same vein, GABA released from neurogliaform cells into the extracellular space also acts on surrounding astrocytes, assigning a role for volume transmission in the control of ionic and neurotransmitter homeostasis.[26] Approximately 78% of neurogliaform cell boutons do not form classical synapses. This may be the first definitive example of neurons communicating chemically where classical synapses are not present.[25]
Relationship to electrical synapses[edit]
An electrical synapse is an electrically conductive link between two abutting neurons that is formed at a narrow gap between the pre- and postsynaptic cells, known as a gap junction. At gap junctions, cells approach within about 3.5 nm of each other, rather than the 20 to 40 nm distance that separates cells at chemical synapses.[27][28] As opposed to chemical synapses, the postsynaptic potential in electrical synapses is not caused by the opening of ion channels by chemical transmitters, but rather by direct electrical coupling between both neurons. Electrical synapses are faster than chemical synapses.[13] Electrical synapses are found throughout the nervous system, including in the retina, the reticular nucleus of the thalamus, the neocortex, and in the hippocampus.[29] While chemical synapses are found between both excitatory and inhibitory neurons, electrical synapses are most commonly found between smaller local inhibitory neurons. Electrical synapses can exist between two axons, two dendrites, or between an axon and a dendrite.[30][31] In some fish and amphibians, electrical synapses can be found within the same terminal of a chemical synapse, as in Mauthner cells.[32]
Effects of drugs[edit]
One of the most important features of chemical synapses is that they are the site of action for the majority of psychoactive drugs. Synapses are affected by drugs, such as curare, strychnine, cocaine, morphine, alcohol, LSD, and countless others. These drugs have different effects on synaptic function, and often are restricted to synapses that use a specific neurotransmitter. For example, curare is a poison that stops acetylcholine from depolarizing the postsynaptic membrane, causing paralysis. Strychnine blocks the inhibitory effects of the neurotransmitter glycine, which causes the body to pick up and react to weaker and previously ignored stimuli, resulting in uncontrollable muscle spasms. Morphine acts on synapses that use endorphin neurotransmitters, and alcohol increases the inhibitory effects of the neurotransmitter GABA. LSD interferes with synapses that use the neurotransmitter serotonin. Cocaine blocks reuptake of dopamine and therefore increases its effects.
History and etymology[edit]
During the 1950s, Bernard Katz and Paul Fatt observed spontaneous miniature synaptic currents at the frog neuromuscular junction.[33] Based on these observations, they developed the ‘quantal hypothesis’ that is the basis for our current understanding of neurotransmitter release as exocytosis and for which Katz received the Nobel Prize in Physiology or Medicine in 1970.[34] In the late 1960s, Ricardo Miledi and Katz advanced the hypothesis that depolarization-induced influx of calcium ions triggers exocytosis.
Sir Charles Scott Sherringtonin coined the word ‘synapse’ and the history of the word was given by Sherrington in a letter he wrote to John Fulton:
‘I felt the need of some name to call the junction between nerve-cell and nerve-cell… I suggested using «syndesm»… He [ Sir Michael Foster ] consulted his Trinity friend Verrall, the Euripidean scholar, about it, and Verrall suggested «synapse» (from the Greek «clasp»).’–Charles Scott Sherrington[4]
See also[edit]
- Acclimatisation (neurones)
- Neuroscience
- Ribbon synapse
Notes[edit]
- ^ Drachman D (2005). «Do we have brain to spare?». Neurology. 64 (12): 2004–5. doi:10.1212/01.WNL.0000166914.38327.BB. PMID 15985565. S2CID 38482114.
- ^ Alonso-Nanclares L, Gonzalez-Soriano J, Rodriguez JR, DeFelipe J (September 2008). «Gender differences in human cortical synaptic density». Proc. Natl. Acad. Sci. U.S.A. 105 (38): 14615–9. Bibcode:2008PNAS..10514615A. doi:10.1073/pnas.0803652105. PMC 2567215. PMID 18779570.
- ^ Brain Facts and Figures Washington University.
- ^ a b Cowan, W. Maxwell; Südhof, Thomas C.; Stevens, Charles F. (2003). Synapses. JHU Press. p. 11. ISBN 9780801871184. Retrieved 9 June 2020.
- ^
Rapport, Richard L. (2005). Nerve Endings: The Discovery of the Synapse. W. W. Norton & Company. pp. 1–37. ISBN 978-0-393-06019-5.
- ^
Squire, Larry R.; Floyd Bloom; Nicholas Spitzer (2008). Fundamental Neuroscience. Academic Press. pp. 425–6. ISBN 978-0-12-374019-9. - ^
Hyman, Steven E.; Eric Jonathan Nestler (1993). The Molecular Foundations of Psychiatry. American Psychiatric Pub. pp. 425–6. ISBN 978-0-88048-353-7.
- ^
Smilkstein, Rita (2003). We’re Born to Learn: Using the Brain’s Natural Learning Process to Create Today’s Curriculum. Corwin Press. p. 56. ISBN 978-0-7619-4642-7.
- ^ a b Lytton, William W. (2002). From Computer to Brain: Foundations of Computational Neuroscience. Springer. p. 28. ISBN 978-0-387-95526-1. Axons connecting dendrite to dendrite are dendrodendritic synapses. Axons which connect axon to dendrite are called axodendritic synapses
- ^ a b
Garber, Steven D. (2002). Biology: A Self-Teaching Guide. John Wiley and Sons. p. 175. ISBN 978-0-471-22330-6.
synapses connect axons to cell body.
- ^ a b
Weiss, Mirin; Dr Steven M. Mirin; Dr Roxanne Bartel (1994). Cocaine. American Psychiatric Pub. p. 52. ISBN 978-1-58562-138-5. Retrieved 2008-12-26. Axons terminating on the postsynaptic cell body are axosomatic synapses. Axons that terminate on axons are axoaxonic synapses
- ^ Widrow, Bernard; Kim, Youngsik; Park, Dookun; Perin, Jose Krause (2019). «Nature’s Learning Rule». Artificial Intelligence in the Age of Neural Networks and Brain Computing. Elsevier. pp. 1–30. doi:10.1016/b978-0-12-815480-9.00001-3. ISBN 978-0-12-815480-9. S2CID 125516633.
- ^ a b Kandel, Schwartz & Jessell 2000, p. 182
- ^ a b c Bear, Mark F; Connors, Barry W; Paradiso, Michael A (2007). Neuroscience: exploring the brain. Philadelphia, PA: Lippincott Williams & Wilkins. pp. 113–118.
- ^
Llinás R, Steinberg IZ, Walton K (1981). «Relationship between presynaptic calcium current and postsynaptic potential in squid giant synapse». Biophysical Journal. 33 (3): 323–351. Bibcode:1981BpJ….33..323L. doi:10.1016/S0006-3495(81)84899-0. PMC 1327434. PMID 6261850. - ^ Chapman, Edwin R. (2002). «Synaptotagmin: A Ca2+ sensor that triggers exocytosis?». Nature Reviews Molecular Cell Biology. 3 (7): 498–508. doi:10.1038/nrm855. ISSN 1471-0080. PMID 12094216. S2CID 12384262.
- ^ Craig C. Garner and Kang Shen. Structure and Function of Vertebrate and Invertebrate Active Zones. Structure and Functional Organization of the Synapse. Ed: Johannes Hell and Michael Ehlers. Springer, 2008.
- ^ Romanov, Roman A.; Lasher, Robert S.; High, Brigit; Savidge, Logan E.; Lawson, Adam; Rogachevskaja, Olga A.; Zhao, Haitian; Rogachevsky, Vadim V.; Bystrova, Marina F.; Churbanov, Gleb D.; Adameyko, Igor; Harkany, Tibor; Yang, Ruibiao; Kidd, Grahame J.; Marambaud, Philippe; Kinnamon, John C.; Kolesnikov, Stanislav S.; Finger, Thomas E. (2018). «Chemical synapses without synaptic vesicles: Purinergic neurotransmission through a CALHM1 channel-mitochondrial signaling complex». Science Signaling. 11 (529): eaao1815. doi:10.1126/scisignal.aao1815. ISSN 1945-0877. PMC 5966022. PMID 29739879.
- ^ a b Sherwood L., stikawy (2007). Human Physiology 6e: From Cells to Systems
- ^ Díaz-Ríos M, Miller MW (June 2006). «Target-specific regulation of synaptic efficacy in the feeding central pattern generator of Aplysia: potential substrates for behavioral plasticity?». Biol. Bull. 210 (3): 215–29. doi:10.2307/4134559. JSTOR 4134559. PMID 16801496. S2CID 34154835.
- ^ a b Rang, H.P.; Dale, M.M.; Ritter, J.M. (2003). Pharmacology (5th ed.). Edinburgh: Churchill Livingstone. p. 129. ISBN 978-0-443-07145-4.
- ^ Bruce Alberts; Alexander Johnson; Julian Lewis; Martin Raff; Keith Roberts; Peter Walter, eds. (2002). «Ch. 11. Section: Single Neurons Are Complex Computation Devices». Molecular Biology of the Cell (4th ed.). Garland Science. ISBN 978-0-8153-3218-3.
- ^ Zoli M, Torri C, Ferrari R, et al. (1998). «The emergence of the volume transmission concept». Brain Res. Brain Res. Rev. 26 (2–3): 136–47. doi:10.1016/S0165-0173(97)00048-9. PMID 9651506. S2CID 20495134.
- ^ Fuxe K, Dahlström A, Höistad M, et al. (2007). «From the Golgi-Cajal mapping to the transmitter-based characterization of the neuronal networks leading to two modes of brain communication: wiring and volume transmission» (PDF). Brain Res Rev. 55 (1): 17–54. doi:10.1016/j.brainresrev.2007.02.009. hdl:10447/9980. PMID 17433836. S2CID 1323780.
- ^ a b Oláh S, Füle M, Komlósi G, et al. (2009). «Regulation of cortical microcircuits by unitary GABA-mediated volume transmission». Nature. 461 (7268): 1278–81. Bibcode:2009Natur.461.1278O. doi:10.1038/nature08503. PMC 2771344. PMID 19865171.
- ^ Rózsa M, Baka J, Bordé S, Rózsa B, Katona G, Tamás G, et al. (2015). «Unitary GABAergic volume transmission from individual interneurons to astrocytes in the cerebral cortex» (PDF). Brain Structure and Function. 222 (1): 651–659. doi:10.1007/s00429-015-1166-9. PMID 26683686. S2CID 30728927.
- ^ Kandel, Schwartz & Jessell 2000, p. 176
- ^ Hormuzdi et al. 2004
- ^ Connors BW, Long MA (2004). «Electrical synapses in the mammalian brain». Annu. Rev. Neurosci. 27 (1): 393–418. doi:10.1146/annurev.neuro.26.041002.131128. PMID 15217338.
- ^ Veruki ML, Hartveit E (December 2002). «Electrical synapses mediate signal transmission in the rod pathway of the mammalian retina». J. Neurosci. 22 (24): 10558–66. doi:10.1523/JNEUROSCI.22-24-10558.2002. PMC 6758447. PMID 12486148.
- ^ Bennett MV, Pappas GD, Aljure E, Nakajima Y (March 1967). «Physiology and ultrastructure of electrotonic junctions. II. Spinal and medullary electromotor nuclei in mormyrid fish». J. Neurophysiol. 30 (2): 180–208. doi:10.1152/jn.1967.30.2.180. PMID 4167209.
- ^ Pereda AE, Rash JE, Nagy JI, Bennett MV (December 2004). «Dynamics of electrical transmission at club endings on the Mauthner cells». Brain Res. Brain Res. Rev. 47 (1–3): 227–44. CiteSeerX 10.1.1.662.9352. doi:10.1016/j.brainresrev.2004.06.010. PMID 15572174. S2CID 9527518.
- ^ Augustine, George J.; Kasai, Haruo (2007-02-01). «Bernard Katz, quantal transmitter release and the foundations of presynaptic physiology». The Journal of Physiology. 578 (Pt 3): 623–625. doi:10.1113/jphysiol.2006.123224. PMC 2151334. PMID 17068096.
- ^ «Nobel prize». British Medical Journal. 4 (5729): 190. 1970-10-24. doi:10.1136/bmj.4.5729.190. PMC 1819734. PMID 4320287.
References[edit]
- Carlson, Neil R. (2007). Physiology of Behavior (9th ed.). Boston, MA: Pearson Education. ISBN 978-0-205-59389-7.
- Kandel, Eric R.; Schwartz, James H.; Jessell, Thomas M. (2000). Principles of Neural Science (4th ed.). New York: McGraw-Hill. ISBN 978-0-8385-7701-1.
- Llinás R, Sugimori M, Simon SM (April 1982). «Transmission by presynaptic spike-like depolarization in the squid giant synapse». Proc. Natl. Acad. Sci. U.S.A. 79 (7): 2415–9. Bibcode:1982PNAS…79.2415L. doi:10.1073/pnas.79.7.2415. PMC 346205. PMID 6954549.
- Llinás R, Steinberg IZ, Walton K (1981). «Relationship between presynaptic calcium current and postsynaptic potential in squid giant synapse». Biophysical Journal. 33 (3): 323–352. Bibcode:1981BpJ….33..323L. doi:10.1016/S0006-3495(81)84899-0. PMC 1327434. PMID 6261850.
- Bear, Mark F.; Connors, Barry W.; Paradiso, Michael A. (2001). Neuroscience: Exploring the Brain. Hagerstown, MD: Lippincott Williams & Wilkins. ISBN 978-0-7817-3944-3.
- Hormuzdi, SG; Filippov, MA; Mitropoulou, G; Monyer, H; Bruzzone, R (March 2004). «Electrical synapses: a dynamic signaling system that shapes the activity of neuronal networks». Biochim Biophys Acta. 1662 (1–2): 113–137. doi:10.1016/j.bbamem.2003.10.023. PMID 15033583.
- Karp, Gerald (2005). Cell and Molecular Biology: concepts and experiments (4th ed.). Hoboken, NJ: John Wiley & Sons. ISBN 978-0-471-46580-5.
- Nicholls, J.G.; Martin, A.R.; Wallace, B.G.; Fuchs, P.A. (2001). From Neuron to Brain (4th ed.). Sunderland, MA: Sinauer Associates. ISBN 978-0-87893-439-3.
External links[edit]
This audio file was created from a revision of this article dated 19 June 2005, and does not reflect subsequent edits.
- Synapse Review for Kids
- Synapse – Cell Centered Database
- Atlas of Ultrastructure Neurocytology An electron microscope picture gallery assembled by Kristen Harris’ lab of synapses and other neuronal structures.
Пресинаптический аппарат:
Это
окончание аксона нейрона (пресинаптическая
терминаль, пресинаптическая бляшка). У
одного аксона может быть множество
пресинаптических терминалей. В этих
терминалях отмечается значительное
скопление митохондрий, указывающее на
активные биосинтетические процессы,
хорошо выражена гладкая эндоплазматическая
сеть, содержащая депонированный кальций,
а также имеется множество микротрубочек
и микрофиламентов, участвующих во
внутриклеточном перемещении мелких
везикул с медиатором. Действительно,
здесь идет активный синтез медиатора
– химического посредника передачи
возбуждения в синапсе, а также упаковка
его в везикулы. В пресинаптическом
аппарате медиатор содержится в везикулах
разного размера. В крупных везикулах
находится запас (депо) медиатора. Эти
везикулы с помощью белка синаптосина
связаны с белками цитоскелета
пресинаптического аппарата и, таким
образом, находятся в фиксированном
состоянии. В мелких везикулах медиатор
находится в молекулярном виде, то есть
он готов к выбросу в синаптическую щель.
В одной такой везикуле находится до 10
тысяч и более молекул медиатора, а размер
везикулы составляет 40-50 нм (рис. 6).
Поверхностная
клеточная мембрана пресинаптического
аппарата, обращенная в синаптическую
щель, называется пресинаптической
мембраной.
В
мионевральном синапсе она имеет
достаточно большую протяженность (около
1-2 мм). Через нее медиатор может проникать
из пресинаптического аппарата в
синаптическую щель.
Синаптическая щель:
Это
пространство между пре- и постсинаптической
мембранами, заполненное межклеточной
жидкостью и содержащее поперечные
гликопротеидные нейрофиламенты
(гликокаликс), идущие от пресинаптической
к постсинаптической мембране. В это
пространство из пресинаптического
аппарата выбрасывается медиатор, который
должен попасть на постсинаптическую
мембрану.
Постсинаптической мембраной:
Постсинаптическая
(иногда ее называют субсинаптической)
мембрана представляет собой участок
поверхностной мембраны клетки (нервной,
мышечной или железистой), на которую
передается возбуждение. Этот участок
находится напротив пресинаптической
мембраны и в отличие от остальной
поверхностной мембраны той же клетки
(например, мышечной) здесь нет «быстрых»
(электровозбудимых) натриевых каналов,
но есть «медленные» — хемовозбудимые,
то есть чувствительные к определенному
медиатору. «Медленные» натриевые каналы
не могут быть открытыми одновременно
все сразу, так как очень быстро происходит
их закрывание (натриевая инактивация).
В связи с вышеизложенным, на постсинаптической
мембране не может возникнуть потенциал
действия, но может появиться местная
деполяризация.
Механизм передачи возбуждения в синапсе
Этот
механизм достаточно сложен. В него
последовательно включаются сначала
пресинаптический аппарат, а потом
постсинаптическая мембрана.
1)
идет перезарядка поверхностной клеточной
мембраны пресинаптического аппарата
(рис. 7А);
2)
ионы кальция по градиенту концентрации
входят в пресинаптический аппарат из
межклеточной жидкости (рис. 7Б);
3)
кальций способствует встраиванию
везикул с медиатором в структуру
пресинаптической мембраны – начальная
фаза экзоцитоза (рис. 7В);
4)
кальций способствует сокращению везикул
и выдавливанию (экзоцитозу) медиатора
в синаптическую щель (рис. 7Г).
Причиной
перезарядки поверхностной клеточной
мембраны пресинаптического аппарата
является возбуждение, поступающее сюда
по аксону и его терминалям от тела
нейрона.
Соседние файлы в предмете [НЕСОРТИРОВАННОЕ]
- #
- #
- #
- #
- #
- #
- #
- #
- #
- #
- #
The Immunological Synapse Part A
Yu Li, Jordan S. Orange, in Methods in Cell Biology, 2023
6 Data analysis
To manually quantify lipid ordering of presynaptic membranes using the Fiji version of ImageJ:
- 1.
-
Download and open software (https://imagej.net/software/fiji/).
- 2.
-
Import and open acquired images with Fiji (at least including three channels: two ratiometric channels for Di-4-ANEPPDHQ probe and one for CellMask deep red).
- 3.
-
Generate binary masks to identify cell membrane region from images of CellMask deep red channel via the Fiji “Threshold” function (via “Image › Adjust › Threshold” or “Ctrl + Shift + T”).
- 4.
-
Calculate GP values from images of the two ratiometric channels via the Fiji “image calculator” function (via “Image › Image Calculator…”). The equation used to calculate the GP value is: GP value = (Di4_A − Di4_B)/(Di4_A + Di4_B)
- 5.
-
Multiply the resulting images with corresponding binary masks to remove background and only preserve the GP values of the membranes of interest via the Fiji “image calculator” function.
- 6.
-
To visualize GP values, choose and apply a lookup table in Fiji and set the display scale manually to “0 to 1” in the “Brightness and Contrast” window. Select a lookup table and range that is consistent with the dynamic range of the GP values to emphasize
- 7.
-
To quantitatively analyze of GP values, obtain measurements via the “Analyze ›Measure” function of Fiji.
- 8.
-
For time-lapse imaging series apply this procedure individually to each frame.
To perform batched imaging analysis automatically using the Fiji version of ImageJ:
- 1.
-
Download and open software (https://imagej.net/software/fiji/).
- 2.
-
Import and open acquired images with Fiji (at least including three channels: two ratiometric channels for Di-4-ANEPPDHQ probe and one for CellMask deep red).
- 3.
-
Rename the images from 500 to 580 nm and 620–750 nm channels as “Di4_A_1, Di4_A_2, Di4_A_3, etc.” and “Di4_B_1, Di4_B_2, Di4_B_3, etc.”, respectively.
- 4.
-
To run auto-analysis script for batch processing, paste and run the source code below via the “Process › Batch › Macro” function of Fiji. User also needs to specify the input directory in the opened dialog window as the location where all Di4 images are located.
Source code of auto-analysis script:
-
selectWindow(«Di4_A.tif»);
-
run(«8-bit»);
-
selectWindow(«Di4_B.tif»);
-
run(«8-bit»);
-
imageCalculator(«Subtract create 32-bit stack», » Di4_A.tif»,» Di4_B.tif»);
-
selectWindow(«Result of Di4_A.tif»);
-
imageCalculator(«Add create 32-bit», «Di4_B.tif»,»Di4_A.tif»);
-
selectWindow(«Result of Di4_B.tif»);
-
imageCalculator(«Divide create 32-bit», » Result of Di4_A.tif»,»Result of Di4_B.tif»);
-
selectWindow(«Result of Di4_A.tif»);
-
close();
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/S0091679X2200098X
Synaptic transmission
Paul Johns BSc BM MSc FRCPath, in Clinical Neuroscience, 2014
Release of neurotransmitter (Fig. 7.4)
Once loaded with neurotransmitter, synaptic vesicles are docked at the presynaptic membrane awaiting release. Docking takes place at active zones. These consist of multi-protein complexes that tether the synaptic vesicle to the presynaptic membrane and contain high concentrations of voltage-gated calcium channels. This leads to brisk calcium influx in response to axon terminal depolarization, since the concentration of calcium is 10,000 times higher in the extracellular fluid. The focal rise in free calcium causes a number of synaptic vesicles to fuse with the presynaptic membrane, emptying their contents into the synaptic cleft by exocytosis.
Release is said to be ‘packeted’ (or quantized) since the total amount of transmitter entering the synaptic cleft is a whole-number multiple of the amount stored in a single vesicle. Transmitter release is regulated by presynaptic autoreceptors which exert negative feedback.
Mechanism of membrane fusion
The mechanism by which synaptic vesicles fuse with the presynaptic membrane is complex and relies on a group of proteins belonging to the SNARE (SNAP receptor) family. The vesicle and presynaptic membranes ‘kiss’ and the interaction between complimentary proteins creates a small fusion pore. This quickly expands as the lipid membranes unite to form a large opening through which the contents of the vesicle are discharged into the synaptic cleft. After exocytosis the vesicle membrane thus becomes part of the presynaptic membrane. However, a similar amount of membrane is reclaimed from the axon terminal to make new vesicles, so there is no net increase in the size of the axon terminal.
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780443103216000072
Advances in Cellular Neurobiology
D.G. Jones, in Advances in Cellular Neurobiology, 1983
B Sequences of Synaptogenesis
The respective sequences of appearance of the pre- and postsynaptic membranes, and the differential roles of the membrane thickenings and synaptic vesicles in the elaboration of the presynaptic contact area are depicted in Fig. 80.
Fig. 8. Five alternative schemes by which synaptic junctions may develop. For details, see text.
When both pre- and postsynaptic densities are present early in development, as in Fig. 8a, they appear as continuous plaques (Aghajanian and Bloom, 1967; Jones and Revell, 1970a,b) which, later in development, undergo gradual differentiation and focalization to produce the typical paramembranous densities of the adult synaptic junction. Early workers repeatedly stressed the presence of membrane thickenings prior to the appearance of synaptic vesicles (Glees and Sheppard, 1964; Hamori and Dyachkova, 1964; Meller, 1964; Bunge et al., 1967; Larramendi, 1969; Tennyson, 1970; Alley, 1973; Stelzner et al., 1973). In all these instances, however, caution is necessary to ensure that the membrane specializations actually are precursors of synaptic junctions rather than early components of desmosome-like contacts. This also applies to the idea that puncta adhaerenta are precursors of synaptic junctions (McGraw and McLaughlin, 1980).
An alternative, therefore, is Fig. 8b with synaptic vesicles preceding membrane thickenings. While the presence of presynaptically located vesicles may not constitute an obligatory feature of immature synapses, it remains a helpful one and should not be lightly discarded. Unfortunately, once the presence of vesicles becomes an essential diagnostic feature of early synapses, it also to some extent prejudges the question of whether vesicles or membrane thickenings appear first in synaptic development.
A third alternative is Fig. 8c, in which increases in vesicle numbers and maturation of the paramembranous densities are simultaneous events (Ochi, 1967; Bodian, 1968; Jones and Revell, 1970a,b). For instance, Jones and Revell noted in rat cerebral cortex that vesicles are present when both synaptic membranes are plaquelike and undifferentiated, suggesting that synaptic vesicles appear with (or even after) undifferentiated membrane thickenings but before their differentiation into recognizable dense projections. Comparable observations have been made in other situations, including chick retina (McLaughlin, 1976) and kitten spinal trigeminal nucleus (Dunn and Westrum, 1978).
The discussion this far has assumed that the pre- and postsynaptic membranes appear concurrently (Aghajanian and Bloom, 1967; Jones and Revell, 1970a,b; Adinolfi, 1972a,b; Bloom, 1972). However, this is far from a universal phenomenon. In some studies, as shown in Fig. 8d, the postsynaptic density appears as a well-established entity before the presynaptic region with its dense projections (Poppe et al., 1973; Jones et al., 1974; West and Del Cerro, 1976; Hinds and Hinds, 1976). This phenomenon is best seen in E-PTA-stained material and has so far been described in developing guinea pig cerebral cortex (Jones et al., 1974) and in some synaptic junctions in fetal rat cerebellum (West and Del Cerro, 1976). It is possible that this appearance reflects features of the E-PTA staining reaction, in that it is not staining the presynaptic components of at least some of the immature synapses. Even if this is the case, however, other evidence points to the legitimacy of “naked” postsynaptic sites during early neuronal development.
The converse arrangement, whereby the presynaptic membrane appears prior to the postsynaptic, is far less frequently encountered (Fig. 8e). A commonly quoted illustration of this is the membrane-vesicle clusters described in the cervical spinal cord of Xenopus laevis embryos (Hayes and Roberts, 1973). A similar sequence of events has been described as part of the pattern for photoreceptor synaptogenesis in developing chick retina (McLaughlin, 1976).
These diverse developmental patterns reflect the range of ways by which pre- and postsynaptic junctional elements establish their adult organization. It is not known whether a particular pattern characterizes a particular synaptic population, or whether similar synapses are put together in different ways under differing environmental pressures.
It has long been argued that axodendritic synapses are formed prior to axo-somatic ones (Voeller et al., 1963; Jones, 1975), the synaptic vesicles being spherical rather than flattened (Oppenheim and Foelix, 1972). More recent studies substantiate these general principles in a wide range of situations, e.g., chick optic tectum (McGraw and McLaughlin, 1980), human cervical spinal cord (Okado, 1980), and mouse spinal motor neurons (Vaughn et al., 1977). Occasional exceptions do occur however, including the study of Smolen and Raisman (1980) on rat superior cervical ganglion. In this instance, axosomatic synapses are present transiently for the first 2 weeks of life. It is possible that such synapses may facilitate dendritic development by providing directive cues to the location of the developing synaptogenic field (Vaughn et al., 1977).
Early axodendritic synapses may be of two types: those with asymmetrical junctions and spherical vesicles, and those with symmetrical junctions and spherical vesicles (Devon and Jones, 1981). Neocortical development is characterized by a decrease in the symmetrical/spherical group. This trend may represent a developmental continuum of symmetrical/spherical to asymmetrical/spherical synapses (Bunge et al., 1967; Cragg, 1972; Hinds and Hinds, 1976; Dunn and Westrum, 1978), although the possibility that the asymmetrical/axodendritic terminals represent an intermediate synaptic type cannot be excluded (Adams and Jones, 1982a). The recognition of the symmetrical/spherical terminal type may provide a means of distinguishing immature terminals in adult, and especially in retarded adult, cortical tissue (Devon and Jones, 1981). In this connection it would be interesting to know whether these symmetrical/spherical terminals correspond to the immature type E synaptic junction described by Dyson and Jones (1976a) in E-PTA-stained cortical material.
Terminals with spherical vesicles are repeatedly described as being the first to appear, with flattened or pleomorphic vesicles appearing in later-developing terminals, e.g., in chick optic tectum (McGraw and McLaughlin, 1980), human cervical spinal cord (Okado, 1980), and rabbit superior colliculus and visual cortex (Mathers et al., 1978).
Quantitative approaches to the morphological categorization of developing synaptic junctions are currently confined to the characterization of E-PTA-stained junctions. The quantitative morphogenetic scheme of Dyson and Jones (1976a) assigns synaptic junctions to five categories, A–E, on the basis of variations in the organization of their presynaptic densities. Of these descriptive characteristics, type A represents a mature junctional form with well-defined and discrete dense projections, whereas, at the other end of the scale, type E is an immature form lacking recognizable presynaptic densities; types B–D probably represent intermediate forms (Fig. 3).
The approach to synaptogenesis illustrated by this study gives some idea of the manner in which synaptic ultrastructure can be traced at varying stages during maturation and adult life. By permitting quantitation of the contribution a particular morphogenetic form makes to the population at developmental intervals, it provides a model system for assessing the maturity of both individual junctions and synaptic populations.
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780120083046500129
Drosophila model of amyotrophic lateral sclerosis targeting FUS and ubiquilin
Masamitsu Yamaguchi, … Hideki Yoshida, in Handbook of Animal Models in Neurological Disorders, 2023
Mini-dictionary of terms
Active zone, a specialized area on the presynaptic membrane of a nerve cell at which synaptic vesicles fuse to release neurotransmitters into the synaptic cleft and transmit signals to the next synapse or muscle.
Amyotrophic lateral sclerosis, a rare neurodegenerative disease that primarily affects the neurons responsible for controlling voluntary muscle movement.
D. melanogaster, a species of fly in the family Drosophilidae.
Frontotemporal lobar degeneration, a clinically and pathologically heterogeneous syndrome, characterized by progressive declines in behavior or language associated with the degeneration of the frontal and anterior temporal lobes.
FUS, the FET family of RNA-binding proteins that regulate gene expression, genomic integrity, and RNA processing.
GAL4-UAS-targeted expression system, a system using the yeast GAL4 protein that interacts with UAS to induce the expression of genes from any organism in tissue- and temporal-specific manners.
Long non-coding RNA, a group of non-coding RNAs longer than 200 nucleotides.
Neuromuscular junction, a synaptic connection between the terminal end of a motor nerve and a muscle.
RNA interference, a post-transcriptional genetic mechanism of eukaryotes that suppresses gene expression and in which double-stranded RNA cleaved into small fragments initiates the degradation of a complementary mRNA sequence.
Ubiquilin, a protein that regulates protein homeostasis (proteostasis).
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780323898331000331
Pharmacology for the Interventional Pain Physician
B. Todd Sitzman M.D., M.P.H., … Honorio Benzon M.D., in Essentials of Pain Medicine and Regional Anesthesia (Second Edition), 2005
Structure and Mechanism of Action of BTs:
BTs are synthesized as single-chain polypeptides and are activated by proteolytic enzymes in a cleaving process (Fig. 18-4). The cleaved heavy chain is responsible for high-affinity docking of the neurotoxin to the presynaptic nerve terminal receptor, enabling the internalization of the bound toxin into the cell.50 The light chain is a zinc-dependent endopeptidase that cleaves membrane proteins responsible for docking acetylcholine vesicles on the inner side of the nerve terminal membrane. The cleavage of these proteins irreversibly precludes fusion of the vesicles with the nerve membrane, thereby preventing release of neurotransmitters into the neuromuscular junction.51
Type A BT appears to be the most potent of the subtypes, and, when injected clinically, has the longest duration of action. While type B and F have limited clinical use, and others are the subject of further study, the multiple differences thus far observed suggest that the subtypes are not interchangeable.
Pharmacological effect of BTs occurs in three stages: binding, internalization, and proteolysis.52
BINDING:
The binding of BTs to the motor endplate presynaptic membrane is a two-stage process.53,54 The C-terminal region of the heavy chain binds in a serotype-specific manner to receptors on the axon terminals of cholinergic neurons. Binding is irreversible and is independent of nerve activity.55 Specific gangliosides have been proposed as the receptors, as well as specific proteins.56,57
INTERNALIZATION:
Binding of the neurotoxin induces the formation of an endosome that carries the toxin into the axon terminal. Internalization of the bound toxin occurs by receptor-mediated endocytosis. Once formed, the contents of the endosome become increasingly acidic, most likely by normal cellular mechanisms. The decrease in pH within the endosome prompts a configurational change in the toxin, which then forms a channel through the membrane. The channel allows all or part of the toxin to enter the cytosol.58 This process is pH dependent.
PROTEOLYSIS (FIG. 18-5):
The exceptional potency of the botulinum neurotoxins has long suggested that a catalytic effect is involved. In the cytosol the proteolytic effects occur. BT types A, E, and C cleave synaptosome-associated protein-25 (SNAP-25). Types B, D, F, and G cleave the synaptic protein synaptobrevin, also known as vesicle-associated membrane protein (VAMP). Type C also cleaves syntaxin.59 Each of these protein substrates participates in the formation of the exocytotic SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) complex, which is essential for fusion of acetylcholine-containing vesicles with the presynaptic membrane, a prerequisite for acetylcholine release. SNAREs form coiled bundles that bridge the membrane of the synaptic vesicle with the plasma membrane by an interaction between VAMP, which is anchored in the vesicle membrane, and syntaxin, which is anchored in the plasma membrane, perhaps preceded by an interaction between VAMP and SNAP-25.60
The effects of the botulinum neurotoxins are due to irreversible inhibition of the release of acetylcholine from cholinergic nerve terminals, including those of motor neurons, preganglionic sympathetic and parasympathetic neurons, and postganglionic parasympathetic nerves.
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780443066511500228
Cytology of the nervous system
Jahangir Moini MD, MPH, … Mohtashem Samsam MD, PhD, in Epidemiology of Brain and Spinal Tumors, 2021
Synapses
Effective messages move along an axon and are transferred to another cell, occurring at a synapse, a specialized area where a neuron communicates with another cell. Synapses are also referred to as synaptic junctions. At a synapse, information moves from the presynaptic neuron to the postsynaptic neuron. Synapses can involve various postsynaptic cells. An example is the neuromuscular junction, a synapse in which the postsynaptic cell is a skeletal muscle fiber. There are more than 100 trillion synapses in the human brain alone. The types of synapses in the CNS are summarized in Table 2.4.
Table 2.4. Types of synapses in the central nervous system.
| Type | Presynaptic Structure | Postsynaptic Structure | Functions |
|---|---|---|---|
| Axodendritic | Axon terminal | Dendrite | Mostly excitatory |
| Axosomatic | Axon terminal | Cell body | Mostly inhibitory |
| Axoaxonic | Axon terminal | Axon terminal | Presynaptic inhibition, which regulates transmitter release in the postsynaptic axon |
| Dendrodendritic | Dendrite | Dendrite | Local interactions that can be excitatory or inhibitory, in neurons that lack axons, such as the retina |
Structure
A synapse consists of three components: the presynaptic membrane formed by the terminal button of an axon, the postsynaptic membrane formed from a segment of dendrite or cell body, and the space in between these two structures, the synaptic cleft. Some neural cells have up to two hundred thousand synaptic connections.
Classification
There are two types of synapses: electrical and chemical, with unique functions. At electrical synapses, there is direct physical contact between cells. Presynaptic and postsynaptic membranes of cells are joined at gap junctions (see Fig. 2.6A). Lipid areas of nearby membranes are separated by just two nanometers (nm), kept in position by essential membrane proteins called connexons. Pores formed by these proteins allow ions to pass between the cells. Changes in membrane potential of one cell cause local currents to affect another. An electrical synapse propagates action potentials between cells efficiently and quickly because of this. In adults, electrical synapses are not common in the CNS or PNS. They occur in brain areas such as the vestibular nuclei, involved in balance, also in the eyes, and in one or several pairs of PNS ganglia (ciliary ganglia). They are also present in embryonic structures.
Figure 2.6. (A) Electrical synapse; (B) Chemical synapse.
In a chemical synapse (Fig. 2.6B), one neuron signals another. This uses the axon terminal of the presynaptic neuron, which sends the message, and the postsynaptic neuron, which receives it. The cells are separated by the narrow synaptic cleft. A presynaptic cell is most often a neuron. Specialized receptor cells create synaptic connections with dendrites. Postsynaptic cells are neurons or other forms of cells. Communications between neurons occur at synapses on dendrites, cell bodies, or along axons of receiving cells. Axoaxonic synapses are between axons of two neurons. Axosomatic synapses have junctions, at an axon terminal of a neuron, and the cell body of another neuron. In an axodendritic synapse, synaptic contact is between an axon terminal of one neuron and a dendrite of another. Chemical synapses are very common compared to electrical synapses. Communications across chemical synapses occur from presynaptic membranes to postsynaptic membranes, and not in the reverse direction.
A neuromuscular junction is a synapse between a neuron and a skeletal muscle cell. At a neuroglandular junction, a neuron regulates activities of a secretory cell. Neurons innervate many other types of cells, such as adipocytes. Axon terminals of presynaptic cells release neurotransmitters into the synaptic cleft. The neurotransmitters are within synaptic vesicles in the axon terminal. Synaptic vesicles contain a small collection, or quanta, of neurotransmitter. When the terminal is depolarized from an action potential of its “parent” axon, there is a calcium influx that leads to phosphorylation of proteins called synapsins. After this phosphorylation, the vesicle links to the presynaptic membrane that faces the synaptic cleft, and the neurotransmitter is released. Another method of neurotransmitter release is based on transporter molecules, which usually act by taking up neurotransmitters from the synaptic cleft.
Each postsynaptic cell has its own type of axon terminal. A round axon terminal is present if a postsynaptic cell is another neuron. At a neuromuscular junction, the axon terminal is of much more complex. Axon terminal structures include mitochondria and thousands of vesicles containing neurotransmitters. Axon terminals reabsorb breakdown molecules of neurotransmitters at the synapse, then reproduce the neurotransmitters. Axon terminals also constantly receive neurotransmitters created by the cell body, along with enzymes and lysosomes, via anterograde flow.
Function of electrical synapses
In electrical synapses, signal transmission occurs as electrical signals, without using molecules. Signals are not modified during transmission. Electrical signals pass over gap junctions. The space between presynaptic and postsynaptic neurons is very small. Signal transmission occurs in both directions. It happens without use of energy and is therefore passive. Signal transmission via electrical synapses is very fast.
Function of chemical synapses
Electrical events trigger release of neurotransmitter release, flooding the synaptic cleft, and binding to receptors on the postsynaptic plasma membrane. This changes membrane permeability, producing graded potentials. The process is similar to operation of neuromuscular junctions. Chemical synapses do not use direct cellular joining, so there is more variation in results. At a chemical synapse, arriving action potentials may or may not release enough neurotransmitter to bring the postsynaptic neuron to threshold.
Cholinergic synapses release acetylcholine (ACh) at all neuromuscular junctions involving skeletal muscle fibers. They release ACh at many CNS synapses, all PNS neuron-to-neuron synapses, and all neuromuscular and neuroglandular junctions in the parasympathetic nervous system. At cholinergic synapses between neurons, a presynaptic cleft lies between presynaptic and postsynaptic membranes. Most ACh in an axon terminal collects in synaptic vesicles, each having thousands of neurotransmitter molecules. One axon terminal may contain a million such vesicles. When an action potential arrives at the presynaptic axon terminal, it depolarizes the membrane, opening its voltage-gated calcium ion channels for a short time. Extracellular calcium ions enter through these calcium channels. The ions attach to the vesicles containing ACh. Attachment of calcium ions to the vesicles causes release of ACh in the synaptic cleft. The ACh is released in groups of nearly 3000 molecules, the average number of molecules in just one vesicle. Release of ACh stops quickly, since active transport removes calcium ions quickly from cytoplasm, in the axon terminal, back to the extracellular space. Ions are pumped out of the cell, or moved to the mitochondria, awaiting another action potential.
ACh binds to receptors on the postsynaptic membrane, depolarizing it. Across the synaptic cleft, ACh diffuses to postsynaptic membrane receptors. The ACh receptors have chemically gated sodium and potassium ion channels. The main response is increased permeability to sodium ions, causing a depolarization in the postsynaptic membrane of about 20 msec. The cation channels move potassium ions outward from the cell. Sodium ions are moved by a stronger electrochemical gradient. A slight depolarization of the postsynaptic membrane occurs, which is a graded potential. The more ACh released at the presynaptic membrane, the more open cation channels in the postsynaptic membrane. Therefore, there is more depolarization. If depolarization brings an adjoining section of excitable membrane such as the initial axon segment to threshold, an action potential occurs in the postsynaptic neuron. The enzyme Acetylcholinesterase removes ACh from the synaptic cleft. The effects of ACh on the postsynaptic membrane are temporary because acetylcholinesterase (also called AChE or cholinesterase) is contained in the synaptic cleft and postsynaptic membrane. About half of all ACh released at the presynaptic membrane is degraded before reaching the postsynaptic membrane receptors. Only about 20 msec are needed for ACh molecules that bind to receptor sites to be broken down. Through hydrolysis, AChE breaks down ACh into acetate and choline. The water-soluble, vitamin-like nutrient choline is easily absorbed by axon terminals, then used to synthesize more ACh, via acetate provided by coenzyme A (CoA). Coenzymes from vitamins are needed for many enzymatic reactions. Acetate moving away from the synapse can be absorbed and metabolized by postsynaptic cells, or by various cells and tissues.
Synaptic delay
A synaptic delay is the time needed for a signal to cross a synapse between two neurons. There is only 0.2−0.5 msec between arrival of an action potential at the axon terminal and its effect upon the postsynaptic membrane. Most of the delay is due to the time needed for calcium ion influx and release of the neurotransmitters. The delay is not due to neurotransmitter diffusion. The synaptic cleft is thin, and neurotransmitters diffuse across it quickly.
If a delay of 0.5 msec occurs, an action potential can travel over 7 cm (about 3 inches) along a myelinated axon. When information is passed down CNS interneurons, increased synaptic delay may exceed propagation time along the axons. When fewer synapses are involved, cumulative synaptic delay is shorter, and responses are faster. The quickest reflexes have one synapse, with a sensory neuron directly controlling a motor neuron.
Synaptic fatigue
Synaptic fatigue, or short-term synaptic depression, is a temporary inability of neurons to fire, and to transmit input signals. Synaptic fatigue is a type of synaptic plasticity, a form of negative feedback. It is mostly presynaptic in its function. Since ACh molecules are recycled, axon terminals do not completely depend upon ACh delivered through axonal transport from the cell body. With intense stimulation, resynthesis and transport mechanisms may be unable to handle neurotransmitter demands. Then, synaptic fatigue occurs. The response of the synapse is weakened until ACh is replenished.
Point to remember
Diphenylhydantoin, antidepressants classified as selective serotonin reuptake inhibitors (SSRIs), and caffeine can affect synaptic transmission. Diphenylhydantoin reduces frequency of action potentials reaching the axon terminal. The SSRIs block serotonin transport into presynaptic cells, increasing stimulation of postsynaptic cells. Caffeine stimulates activity of the nervous system by lowering synaptic thresholds, causing postsynaptic neurons to be excited more easily.
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780128217368000121
Nicotine
Tursun Alkam, … Toshitaka Nabeshima, in Reference Module in Biomedical Sciences, 2022
Future directions
Nicotine’s toxicity is mediated by nAChR subunits located in pre- or postsynaptic membranes. In addition to membrane internalization, nAChRs have a half-life of up to 10 days when membrane-bound (Benowitz et al., 2009; Benowitz et al., 1982). Nicotine exerts its various toxicities through different subunits of nAChRs, which are located in the pre- or post-synaptic membranes. Nicotine increases the expression and half-life of surface membrane nAChRs (Kuryatov et al., 2005; Nashmi et al., 2003). However, the process or mechanism involved in the degradation or elimination of nAChRs-binding or free nicotine in the synaptic cleft, as well as its half-life, is unknown. The role of nAChRs subunits in nicotine intoxication in various nervous systems is unclear. Future research should focus on synaptic nicotine degradation and nAChR subunits involved in nicotine toxicity.
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780128243152001482
Assembly of Synapses in the Vertebrate Central Nervous System
LEORA GOLLAN, PETER SCHEIFFELE, in Protein Trafficking in Neurons, 2007
C Nucleation of Synaptic Scaffolds by Adhesion Molecules
The mechanical stability of synapses and the precise apposition of pre- and postsynaptic membrane domains implies that adhesion molecules might bridge the synaptic cleft and thereby keep both sides of the synapse in register. Ultrastructural studies revealed the presence of different sites of cell-cell adhesion at the synapse—including the linkage across the synaptic cleft, as well as at puncta adherentia that flank the synaptic cleft. A third site of cellular adhesion at central synapses are the end feet of glial cells that tightly enclose the synaptic junctions.
Most attention in recent years has been focused on neuronal adhesion molecules that directly bridge the synaptic cleft and that might instruct the assembly of synaptic structures. Several such adhesion molecules have been identified. The synaptogenic activity of these adhesive factors is thought to rely on the nucleation of cytoplasmic scaffolds at the pre- and postsynaptic side, which subsequently facilitate the recruitment of other cytoplasmic and cell surface molecules to an incipient synaptic connection.
In vitro experiments with cultured neurons revealed that exposing axonal or dendritic processes to specific adhesive triggers is sufficient to induce pre- and postsynaptic structures. One extensively studied adhesion system with synapse-inducing function is the neuroligin-neurexin complex. Neuroligins and neurexins are heterophilic adhesion proteins that form a trans-synaptic complex (Ichtchenko et al. 1995; Nguyen and Südhof 1997; Song et al. 1999). Cytoplasmic sequences in neuroligin-1 target this protein exclusively to the synaptic sites in dendrites and ensure exclusion from the axons (Dresbach et al. 2004; Iida et al. 2004; Rosales et al. 2005). This strict polarity of neuroligin-1 provides this heterophilic adhesion system with the required directionality for the assembly of asymmetric synaptic structures. Contact of axons with neuroligin-1 ectopically expressed in a nonneuronal cell induces clustering of axonal neurexins and the cytoplasmic scaffolding molecule CASK (Scheiffele et al. 2000; Dean et al. 2003). These neuroligin-induced axonal contacts then mature into functional presynaptic release sites, including active zone components and clusters of synaptic vesicles. Conversely, contact of dendrites with neurexin-1 expressing cells leads to the formation of neuroligin clusters and postsynaptic structures, such as the accumulation of the scaffolding protein PSD95 and NMDA receptors (Graf et al. 2004). These experiments suggest that the neuroligin-neurexin adhesion system can nucleate pre- and postsynaptic structures. To a large extent these structures are thought to assemble around scaffolding components that bind to the cytoplasmic tails of neuroligins and neurexins (see Figure 4.1). For example, postsynaptic neuroligin-1 binds to PSD95, which in turn can recruit NMDA-receptors and other scaffolding components such as GKAP, Homer, and Shank (Irie et al. 1997; Meyer et al. 2004; Prange et al. 2004; Chih et al. 2005).
Interestingly, the neuroligin-2 isoform, which differs in its cytoplasmic sequences from neuroligin-1, is found primarily at GABAergic synapses. Extracellular aggregation of neuroligin-2 results in not only the recruitment of PSD95, but also of gephrin, a scaffolding protein specific to inhibitory synapses (Graf et al. 2004; Varoqueaux et al. 2004). Selective interaction of excitatory and inhibitory postsynaptic scaffolding proteins with different neuroligin cytoplasmic tail sequences therefore might contribute to the nucleation of specific postsynaptic scaffolds. However, interactions between extracellular domains that may contribute to this process have not been ruled out.
Similar experiments revealed synaptogenic activities for the cell adhesion molecule SynCAM, a homophilic Ig-domain protein (Biederer et al. 2002). Interestingly, SynCAM and neuroligin-1 differ in their cytoplasmic interactions with scaffolding proteins. These differences may explain the differential recruitment of NMDA and AMPA-type glutamate receptors to neuroligin-1- and SynCAM-induced synapses, respectively (Sara et al. 2005). Neuroligin-1 recruits PSD95 and NMDA-receptors through the type I PDZ-binding motif in neuroligin, whereas SynCAM contains a type II PDZ-binding motif that contributes to the recruitment of functional AMPA-receptors to synapses. The importance of these selective scaffolding interactions was confirmed in experiments showing that a chimaeric protein, which includes neuroligin-1 containing the cytoplasmic sequences of SynCAM, is capable of stimulating AMPA receptor recruitment (Sara et al. 2005).
Another presumptive cell adhesion molecule that has been specifically linked to the synaptic recruitment of AMPA receptors through interaction with scaffolding molecules is Dasml (Shi et al. 2004). However, the extracellular binding partners of this protein are currently unknown.
It is likely that several adhesion molecules that act through mechanisms similar to the neuroligin-neurexin complex and SynCAM will cooperate in the nucleation of synaptic scaffolds and the subsequent recruitment of neurotransmitter receptors. Candidate molecules include the nectins, a family of homophilic Ig-domain proteins that are similar to SynCAM (Satoh-Horikawa et al. 2000; Mizoguchi et al. 2002). The integration of multiple adhesive signals likely occurs at the level of the cytoplasmic scaffold. There is substantial crosstalk between different scaffolding components; for example, the CASK/Mint/MALS complex that binds to neurexins is linked to beta-catenin, the cytoplasmic binding partner of cadherins (see Figure 4.1; Hata et al. 1996; Perego et al. 2000; Bamji et al. 2003). Such crosstalk might be employed for the sequential recruitment of different adhesive factors to a growing adhesion site as has been observed for cadherins and nectins in epithelial cells (Takahashi et al. 1999; Tachibana et al. 2000).
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780123694379500074
Neurotransmitter release
Constance Hammond, … Michael Seagar, in Cellular and Molecular Neurophysiology (Fourth Edition), 2015
When does Ca2+ enter the presynaptic element in response to a presynaptic spike?
In order to record the presynaptic Ca2+ current, the presynaptic membrane is clamped at VH = −80 mV by means of two whole-cell electrodes. To isolate the presynaptic Ca2+ current, voltage-dependent Na+ and K+ currents are blocked with TTX and tetraethlyammonium chloride (TEA), respectively. An action potential waveform is injected into the presynaptic terminal through one of the whole-cell electrodes. It evokes a presynaptic Ca2+ current that is recorded by the second whole-cell electrode. Recordings show that Ca2+ influx is tightly associated with the repolarizing phase of the action potential (Figure 7.4): it is essentially a tail current (see Appendix 5.2) that activates shortly after the peak of the action potential and ends before repolarization is complete. It has a peak amplitude of 2.6 ± 0.2 nA and a half-width of about 350 ms. The delay between the beginning of the action potential and that of Ca2+ current is about 500 μs at 23–24°C.
Figure 7.4. Ca2+ current flows into presynaptic terminal during the repolarizing phase of the presynaptic spike.
Two whole-cell electrodes are positioned in the calyx of Held; one measures the membrane potential (V) and the other one injects current (I). It is a two-electrode voltage clamp configuration. The preparation is bathed in 2 mM external Ca2+ with TTX and TEA to block the voltage-gated Na+ and K+ currents. (a) The voltage clamp command (VCmd) is an action potential waveform (V) from a holding potential of −80 mV. A reduced and inverted action potential waveform is also applied, scaled and re-inverted to measure the passive current. Top, recorded voltages. The two action potential waveforms are superimposed. Due to series resistance, the repolarization is somewhat slower during the full action potential than during the scaled action potential. Middle, currents. The current flowing during the full-sized action potential has a larger inward component (labeled ICa). The two passive transients overlay well. Bottom, calcium current. The calcium current is obtained by subtracting the passive current from the current measured during the full action potential. All traces are the average of 11. Vertical dotted lines denote peak of the action potential waveform and of the calcium current. (b) This Ca2+ current is reduced in 1 or 0.5 mM external Ca2+ ([Ca2+ ]o)
Part (a) from Borst JG, Sakmann B (1998) Calcium current during a single action potential in a large presynaptic terminal of the ra t brainstem. J. Physiol. 506, 143–157, with permission. Part (b) adapted from Borst JGG, Sakmann B (1996) Calcium influx and transmitter release in a fast CNS synapse. Nature 383, 431–434, with permission.
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780123970329000078
The Neuromuscular Junction and Excitation-Contraction Coupling
Joseph Feher, in Quantitative Human Physiology, 2012
Acetylcholine Is Degraded and Then Recycled
Each action potential fuses perhaps 300 or so synaptic vesicles with the presynaptic membrane. Continuous excitation and subsequent fusion of these large numbers of vesicles with the presynaptic membrane would expand the area of the membrane and deplete it of vesicles. To avoid this, the presynaptic cell rapidly recycles the vesicles. Through an elaborate mechanism involving specialized proteins such as clathrin and dynamin, vesicles are pinched off and endocytosed. The empty vesicles are refilled with acetylcholine through a transport channel that exchanges acetylcholine for H+ ions. The H+ ions are accumulated within the vesicles through a vacuolar H-ATPase in the vesicle membrane.
Acetylcholine is also partially recycled. Acetylcholine is degraded to acetate and choline in the synapse through the action of acetylcholinesterase. The acetate is not recycled, but the choline is taken back up into the presynaptic cell through a transporter on the presynaptic cell membrane. In the cell, acetylcholine is resynthesized from acetyl CoA and choline through the enzyme choline acetyltransferase. The events occurring in the axon terminal that initiate and shut off neuromuscular transmission are shown in Figure 3.6.4.
Figure 3.6.4. Release of neurotransmitter at the neuromuscular junction. The action potential propagates along the axon and opens voltage-gated Ca2+ channels, leading to an increase in cytoplasmic [Ca2+] in the terminal. The [Ca2+] binds to proteins that dock synaptic vesicles at the active zone, leading to fusion of the vesicles with the presynaptic membrane and release of neurotransmitter, acetylcholine, into the synapse. ACh diffuses across the gap to bind to ACh receptors located on the postsynaptic membrane. ACh binding to this receptor increases the K+ and Na+ conductance, which in turn depolarizes the muscle cell membrane. This depolarization is called the end-plate potential. It is conveyed passively to a nearby patch of muscle membrane where, if the end-plate potential is sufficient, it initiates an action potential. Vesicle release is shut off by removal of the synaptic Ca2+ by Ca2+ pumps or Na+/Ca2+ exchange. The acetylcholine signal is terminated by hydrolysis of acetylcholine in the synapse by acetylcholinesterase. VDCC=voltage-dependent calcium channel.
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780123821638000293